All measurement has a limit of precision and accuracy, and this must be taken into account when evaluating experimental results.
- Qualitative data includes all non-numerical information obtained from observations, not from measurement.
- Quantitative data are obtained from measurements and are always associated with random errors/uncertainties determined by the apparatus and by human limitations such as reaction times.
Uncertainty and error in measurement
- Random errors are caused by:
- the readability of the measuring instrument
- the effects of changes in the surrounding such as temperature variations and air currents
- insufficient data
- the observer misinterpreting the reading.
- Random errors make a measurement less precise, but not in any particular direction. They are expressed as an uncertainty range, such as $\rm 25.05 \pm 0.05°C$.
- The uncertainty of an analogue scale is $\pm$ (half the smallest division).
- The uncertainty of a digital scale is $\pm$ (the smallest scale division).
- Systematic errors occur when there is an error in the experimental procedure:
Measuring the volume of water from the top of the meniscus rather than the bottom, or overshooting the volume of a liquid delivered in a titration will lead to readings which are too high.
Heat losses in an exothermic reaction will lead to smaller observed temperatures changes. - Experiments are repeatable if the same person duplicates the experiment with the same results.
- Experiments are reproducible if several experimentalists duplicate the results.
- The precision or reliability of an experiment is a measure of the random error. If the precision is high then the random error is small.
- The accuracy of a result is a measure of how close the result is to some accepted or literature value If an experiment is accurate then the systematic error is very small.
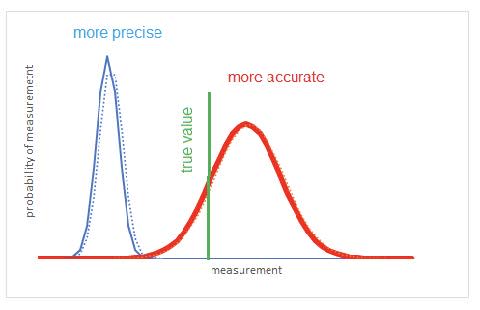
- Random uncertainties can be reduced by repeating readings; systematic errors cannot be reduced by repeating measurements.
- Precise measurements have small random errors and are reproducible in repeated trials. Accurate measurements have small systematic errors and give a result close to the accepted value.
- The number of significant figures in any answer should reflect the number of significant figures in the given data.
- When data is multiplied or divided the answer should be quoted to the same number of significant figures as the least precise.
- When data is added or subtracted the answer should be quoted to the same number of decimal places as the least precise value.
- When adding or subtracting measurements the total absolute uncertainty is the sum of the absolute uncertainties
- When multiplying or dividing measurements the total percentage uncertainty is the sum of the individual percentage uncertainties. The absolute uncertainty can then be calculated from the percentage uncertainty.
- If one uncertainty is much larger than others, the approximate uncertainty in the calculated result can be taken as due to that quantity alone.
- The experimental error in a result is the difference between the recorded value and the generally accepted or literature value.
- Percentage uncertainty = (absolute uncertainty /measured value) $\times 100\%$.
- Percentage error = ((accepted value – experimental value)/ accepted value) $\times 100\%$.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
