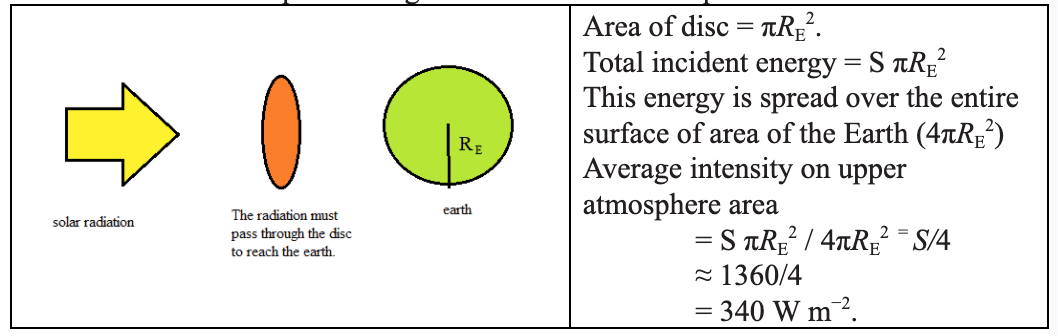
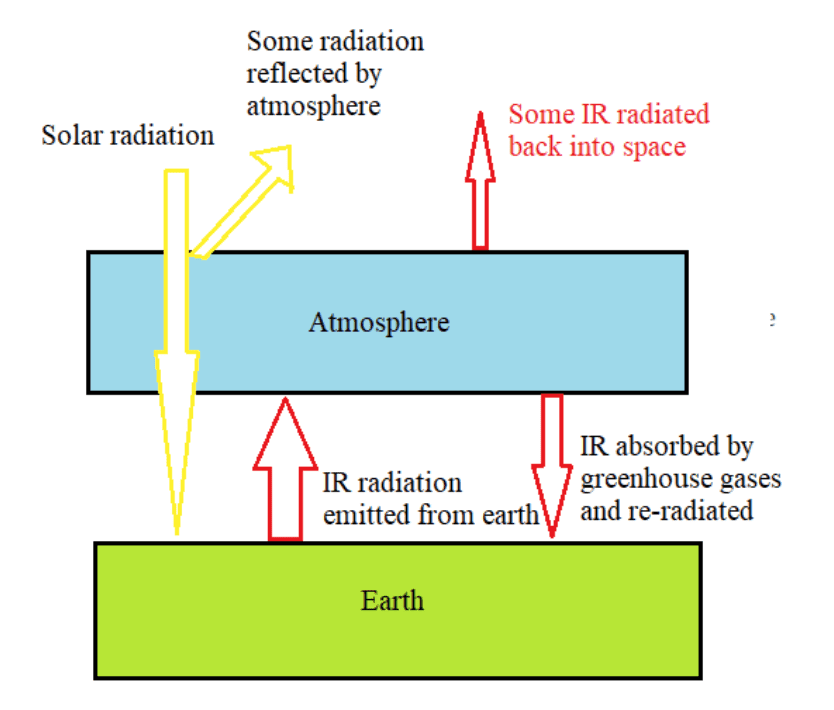
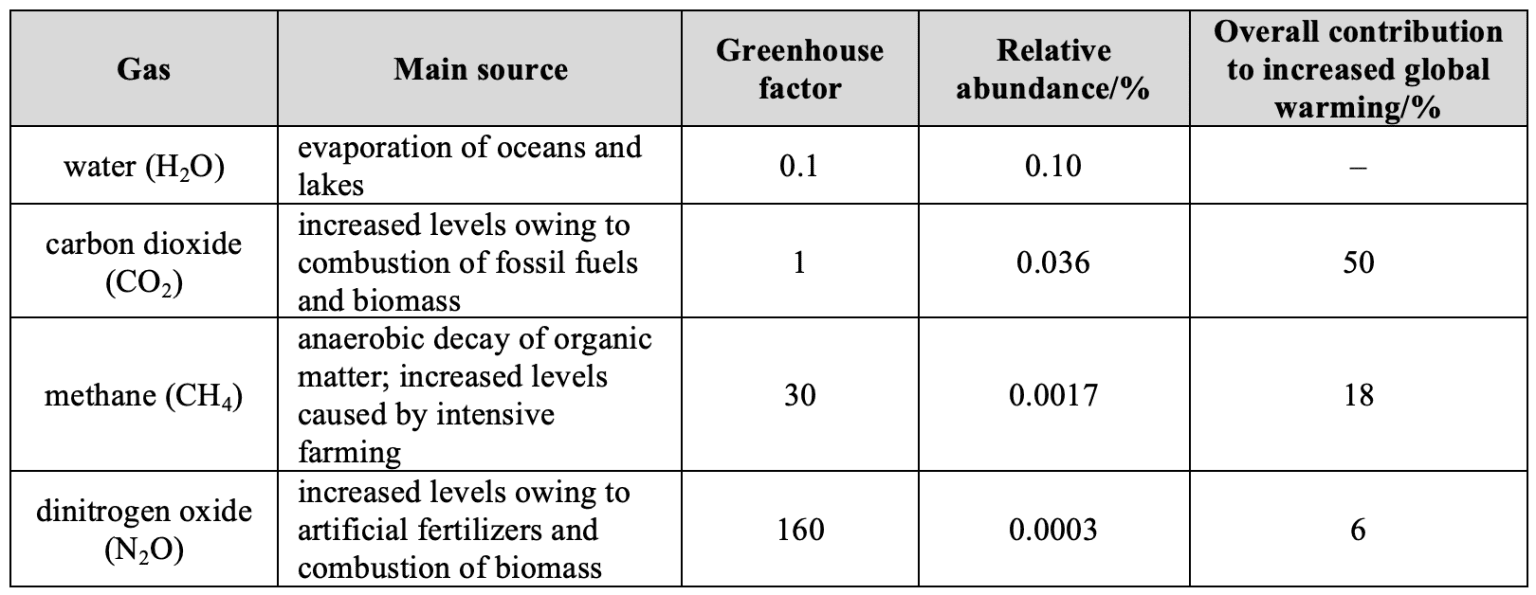
The energy transfer of the earth can be modelled by treating the earth as a black-body radiator and the atmosphere treated as a grey-body.
- Thermal Energy can be transferred by three distinct methods: conduction, convection and radiation.
- Energy can be conducted through solids by lattice vibrations, and the motion of free electrons.
- The rate of flow of energy $(\Delta \mathrm Q/\Delta t)$ through a solid depends on the temperature difference $\rm(\Delta T)$, the length $(l)$ of the piece of solid, the cross-sectional area $\rm (A)$ of the piece of solid and the material.
$\Delta \mathrm Q/\Delta t = k \mathrm A \Delta \mathrm T/l$.
$k$ is the thermal conductivity of the material. - Gases (and many liquids) have low thermal conductivity. Thermal energy can instead be transferred by convection currents due to the movement of gas and liquid particles. Areas at a higher temperature have a lower density than areas at a lower temperature and molecules from these areas move upward carrying thermal energy.
- Convection cannot take place in a solid as the particles have a fixed position.
- Matter is not involved in the transfer of thermal energy by radiation.
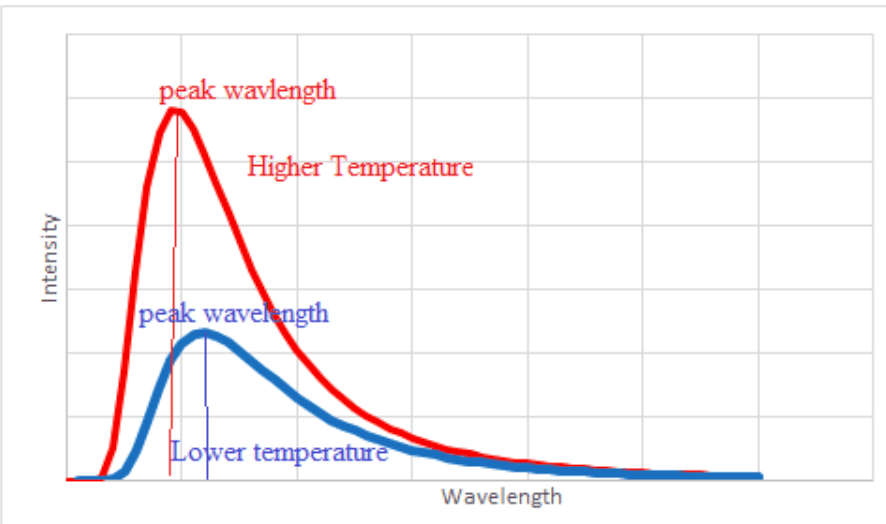
- All bodies emit energy in the form of electro-magnetic radiation. The rate of emission of energy depends on the temperature of the body and the nature of its surface. Most objects emit in the infra-red region of the electromagnetic spectrum.
- Similarly, all bodies absorb electro-magnetic radiation. The rate of absorption depends on the nature of the surface only.
- Dark coloured or dull surfaces both emit and absorb radiation better than light coloured or shiny surfaces.
- Radiation is the only method by which thermal energy van be transferred through a vacuum.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA