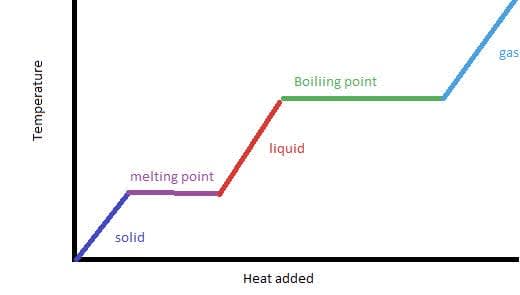
- Solids have a fixed shape and volume.
- Liquids have a fixed volume but not fixed shape.
- Gases have no fixed shape or volume.
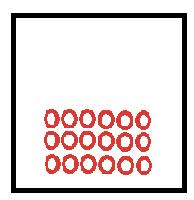 |
Particles in a solid vibrate about fixed position as held together by attractive forces. |
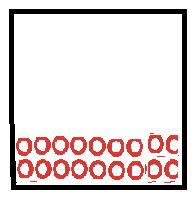 |
Particles in a liquid move in fixed volume as particles held closely together by attractive forces. |
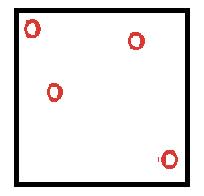 |
Particles in a gas can move freely as negligible attractive forces |