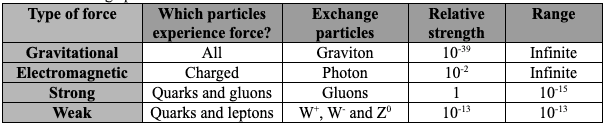
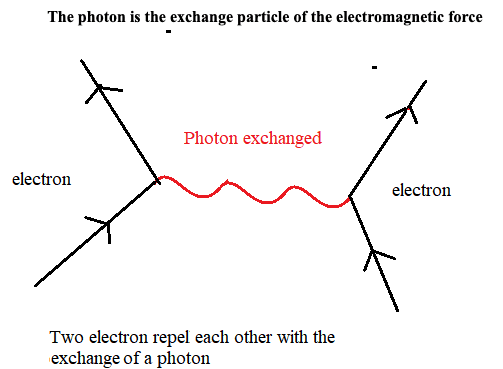
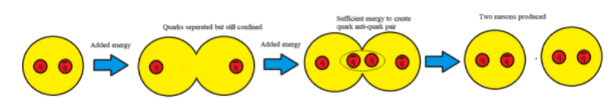
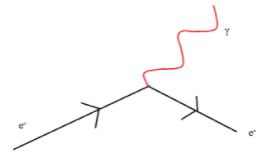
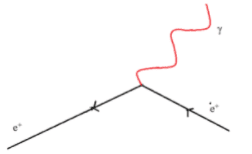
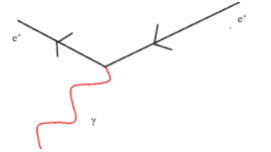
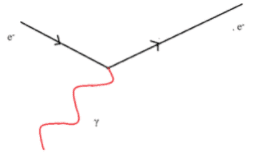
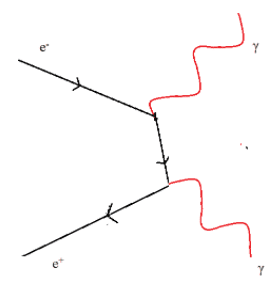
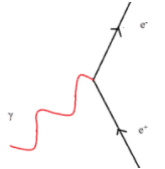
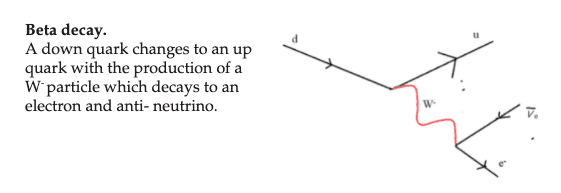
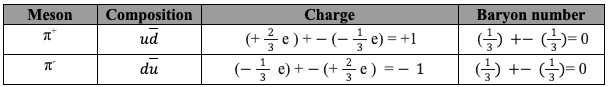All matter is made from fundamental particles called quarks and leptons. Matter has a hierarchical structure: quarks make nucleons, nucleons make nuclei, nuclei and electrons make atoms and atoms make up molecules.
- The atom consists of the three subatomic particles:
Subatomic particle Position Charge / $\bf e*$ Mass / amu Protons Nucleus $\rm +e$ $\sim 1$ Neutrons Nucleus $0$ $\sim 1$ Electrons Outside the nucleus. Gives the atoms its volume. $\rm -e$ $1/1836 \approx 0$
* e is the charge of the electron
- Evidence for the existence of the small, massive, positively charged nucleus in the centre of the atom came from the Rutherford, Marsden, Geiger experiment.
- Alpha particles (massive positively charged particles) from a radium source were directed at a thin gold foil. Most of the alpha particles passed through undeflected but a small number were scattered by small angles, and a very small number even bounced back.
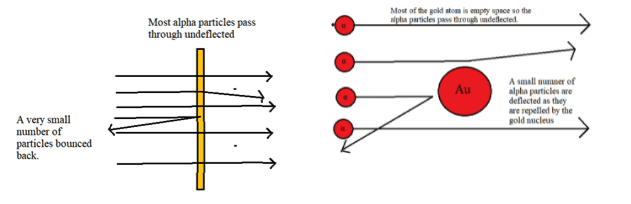
- The results show that the atom is mostly empty space but has a dense positively charged nucleus at its center. There is electrostatic repulsion between the positive charge on the alpha particles and the positive charge in the nucleus.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA