Stereoisomerism involves isomers which have different arrangements of atoms in space but do not differ in connectivity or bond multiplicity (i.e. whether single, double, or triple) between the isomers themselves.
- Stereoisomers differ in the spatial/three-dimensional arrangement of the atoms in a molecule.
- Conformational isomers interconvert by free rotation about a $\sigma$-bond. They are difficult or impossible to isolate as individual compounds, as they quickly interconvert by free rotation about a $\sigma$-bond.
- Configurational isomers interconvert only by breaking and reforming a bond and so have a permanent difference in their geometry. They include cis-trans, $\rm E/Z$ and optical isomers.
cis-trans isomers and $\bf E/Z$ isomers
- Cis-trans isomerism occurs in alkenes and cycloalkanes, where the two substituents at the carbon atoms can be positioned either on the same side (cis-isomer) or opposite sides (trans-isomer) of the $\rm C=C$ bond or the plane of the ring.
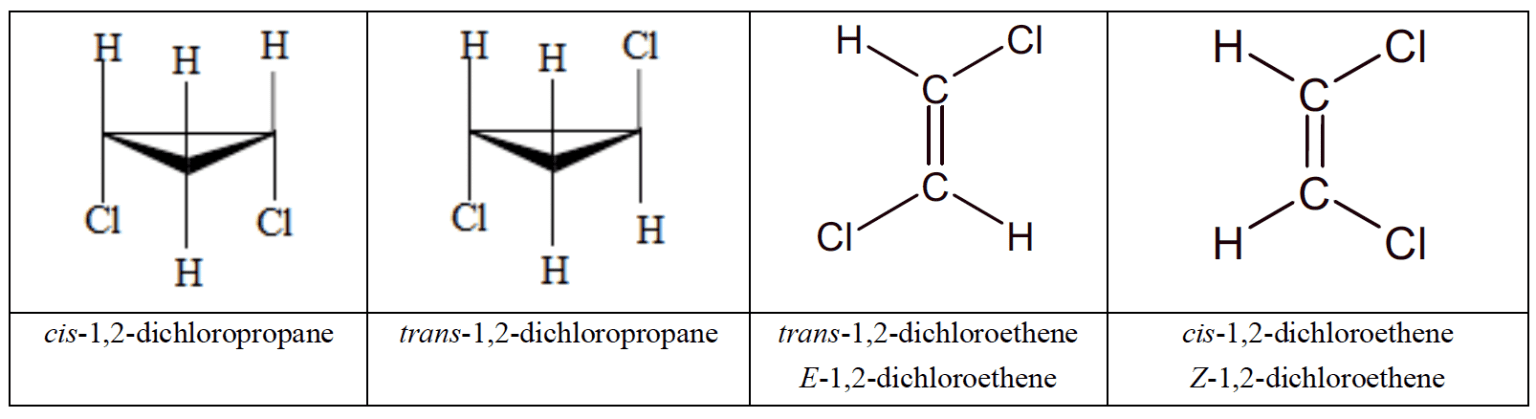
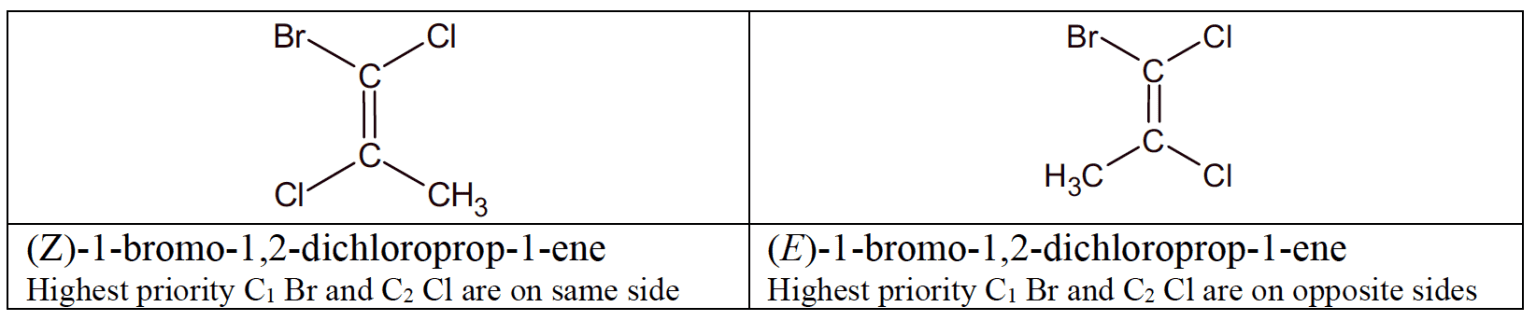
- The $\bf E/Z$ nomenclature has a broader application and must be used when there are more than two different substituents.
- Each group attached to each $\rm C$ atom of the ring or double bond is assigned a priority based on atomic number. The $\rm E$ isomer has the two groups of highest priority on the same side of the double bond or ring, and the $\rm Z$ group has them on the opposite sides.
- "$\rm E$" comes from German Entgegen ("in opposition to"),"$\rm Z$" comes from German Zusammen ("together").
- These isomers generally have different physical properties as the molecules have different polarities.
- The isomers generally have similar chemical properties except in the case when groups on neighbouring carbons can interact in some orientations but not in others.
Optical isomers
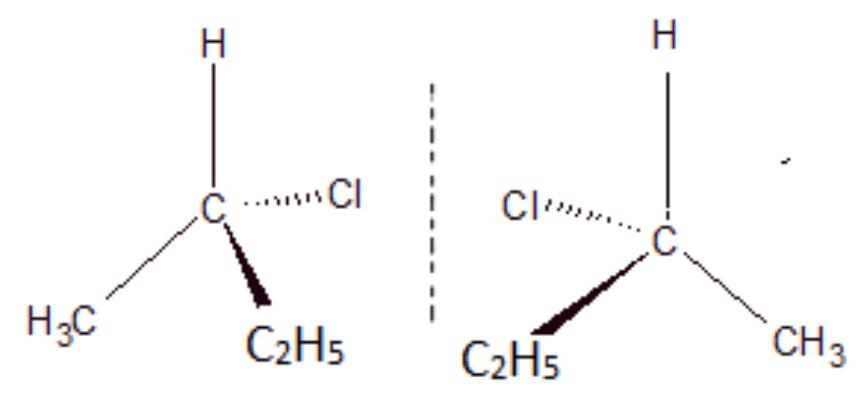
- A chiral or asymmetric carbon atom is joined to four different atoms or groups of atoms.
- A chiral or asymmetric carbon atom and gives rise to enantiomers that are non-superimposable mirror images of each other.
- A polarimeter is an instrument that measures the angle of rotation of plane-polarized light.
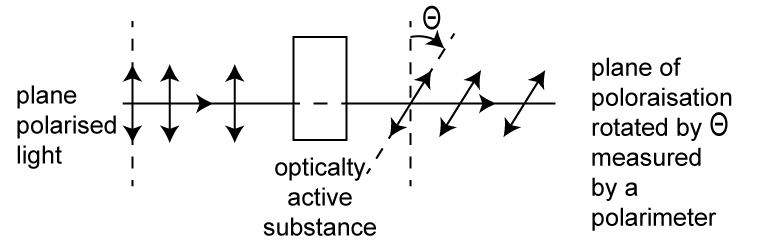
- Optical isomers or enantiomers have identical physical properties except that they rotate the plane of polarized light in opposite directions by the same angle.
- Optical isomers or enantiomers also differ from each other in their reactivity with other chiral molecules. This different reactivity is important in living cells, as all biochemical reactions are stereospecific.
- In biological and medical contexts one enantiomers can be beneficial for the organism while the other can be toxic or responsible for unwanted side effects.
- A racemic mixture contains equal amounts of the two enantiomers and is optically inactive as the effects of individual enantiomers cancel.
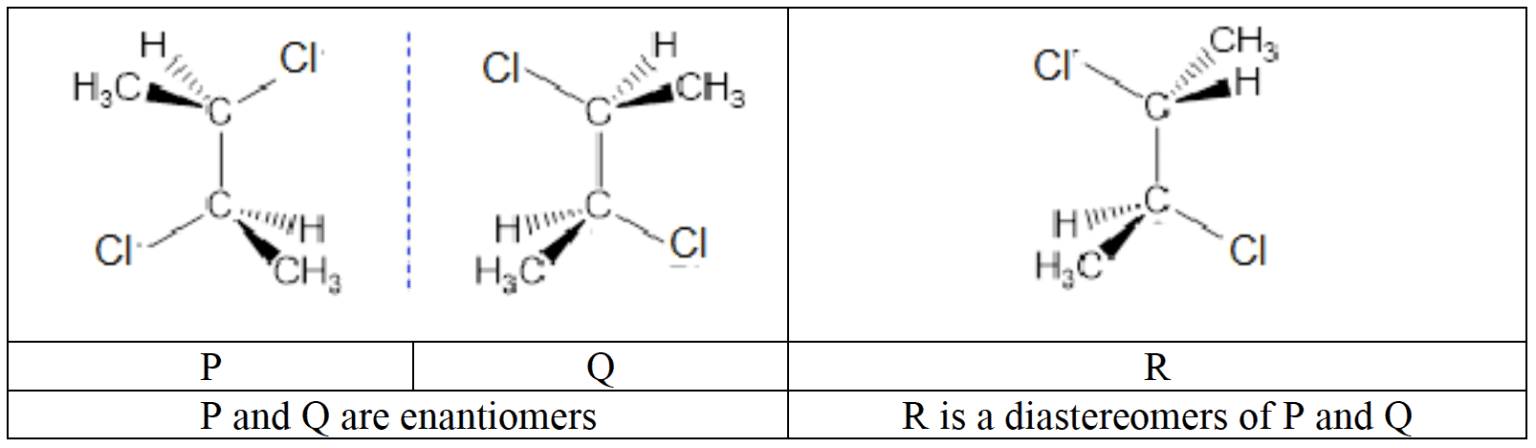
- Diastereoisomers arise when a molecule has more than one chiral centre, and has different configurations in some but not all of these positions. Diastereoisomers are not mirror images of each other and in contrast to enantiomers, diastereomers differ in both physical and chemical properties.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
