Analytical techniques can be used to determine the structure of a compound, analyse the composition of a substance, or determine the purity of a compound. Spectroscopic techniques are used in the structural identification of organic and inorganic compounds.
- Mass spectrometry $\bf (MS)$, proton nuclear magnetic resonance spectroscopy $\bf (^1H ~NMR)$ and infrared spectroscopy $\bf (IR)$ are techniques that can be used to help identify and to determine the structure of organic compounds.
- The IB data booklet contains characteristic ranges for $\rm IR$ absorptions, $\rm ^1H~NMR$ data, specific $\rm MS$ fragments and the formula to determine $\rm IHD$.
- The degree of unsaturation (or index of hydrogen deficiency (IDH)) provides a measure as to the degree of unsaturation of an organic molecule. It relates to how many molecules of hydrogen would in theory be needed to convert an unsaturated molecule to a saturated molecule.
- The $\rm IHD$ can be deduced by identifying the multiple bonds and ring structures in a structure.
- It can also be deduced by comparing the formula with that of the corresponding open chain saturated compound. This can be expressed as an equation for the formula $\rm (CcHhNnOoXx)$ as the equation:
$\mathrm{IHD} = ½ (2c + 2 – h – x + n)$
Worked Example
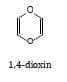
Deduce the $\rm IHD$ for 1,4-dioxin.
Solution
Using the structure:
There is a ring and $\rm 2 C = C$ bonds: The $\rm IHD = 1 + 2 = 3$
The formula $\rm = C_4H_4O_2$
The corresponding saturated compound is $\rm C_4H_{10}O_2: IHD = ½ \times 6 = 3$
Using the equation:
$\mathrm{IHD} = ½ (2c + 2 – h – x + n) = ½ (2 \times 4 + 2 – 4) = ½ \times 6 = 3$
Mass spectrometry
- Molecules are ionised by fast-moving electrons, to form a positive ion. The ion can then fragment into smaller charged and uncharged particles. The largest fragment is the parent ion. Only charged particles are detected.
- A fragmentation pattern can provide evidence for the structure of the compound.
Infrared spectroscopy
- Radiation needed to excite molecules and make them vibrate more occurs in the infrared region of the electromagnetic spectrum.
- $\rm IR$ radiation can cause a bond to stretch or bend. Stretching a bond requires more energy than bending a bond and generally occurs at higher wavenumbers.
- Absorption of particular wavenumbers of IR radiation corresponds to particular bonds:
- Bonds with atoms of small mass absorb at higher wavenumbers than atoms with larger mass.
- Double bonds occur at higher wavenumbers than single bonds.
- Characteristic absorption bands can be found in the IB data booklet.
- Hydrogen bonding broadens the absorptions.
- The intensity of the absorption depends on the polarity of the bond. For a molecule to absorb IR there must be a change in dipole moment during the stretching or bending.
- In a polyatomic molecule such as carbon dioxide it is more correct to consider the molecule stretching and bending as a whole, rather than considering the individual bonds.
- In carbon dioxide, for example, there are four modes of vibration. The symmetric stretch is $\rm IR$ inactive as it produces no change in dipole moment.
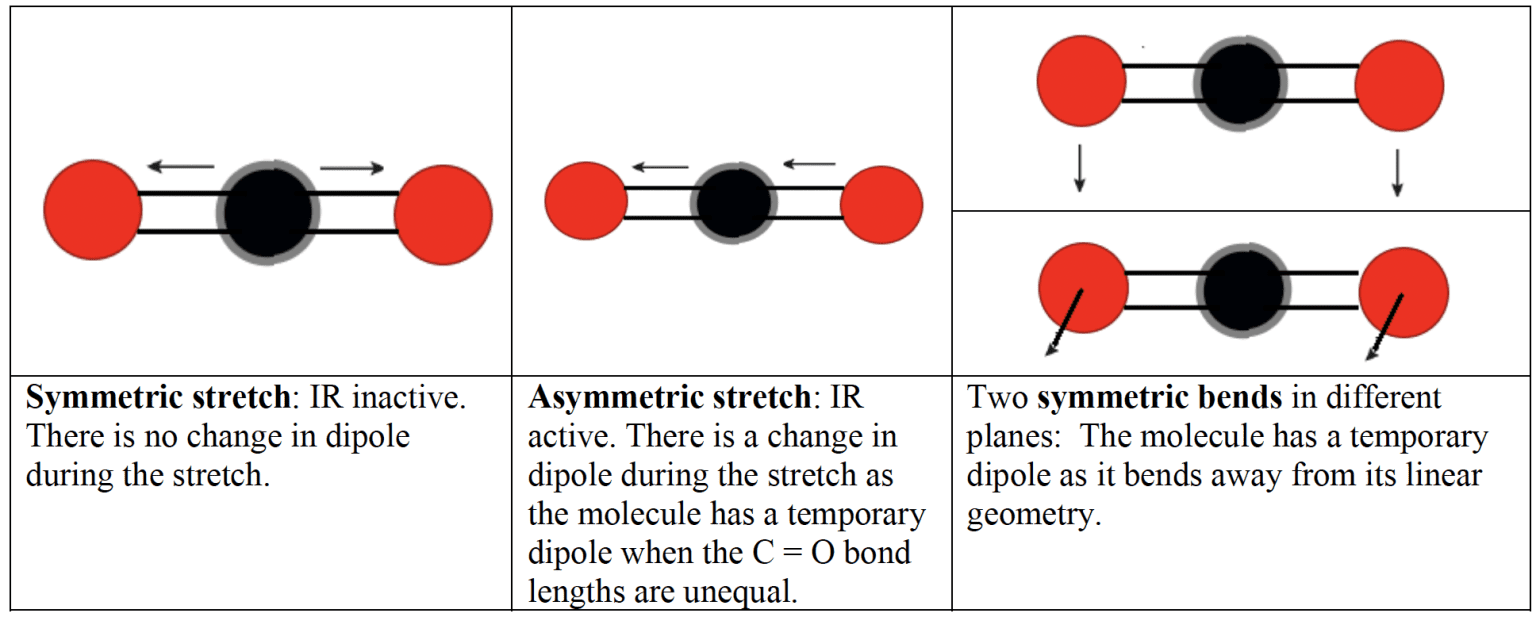
- Molecules with several bonds can vibrate in different ways and with different frequencies. The complex pattern can be used as a fingerprint to be matched against the recorded spectra of known compounds in a database. A comparison of the spectrum of a sample with that of a pure compound can also be used as a test of purity.
Nuclear magnetic resonance spectroscopy
- Some nuclei $\rm (^1H, ^{13}C, ^{19}F,$ and $\rm ^{31}P)$ behave like small magnetics and so can align themselves parallel or in the reverse direction in an external magnetic field. The proton of $\rm ^1H$ is the nuclei generally studied in $\rm NMR$.
- The energy difference between the two orientations is sensitive to the chemical environment of the proton and so is a useful source of structural information.
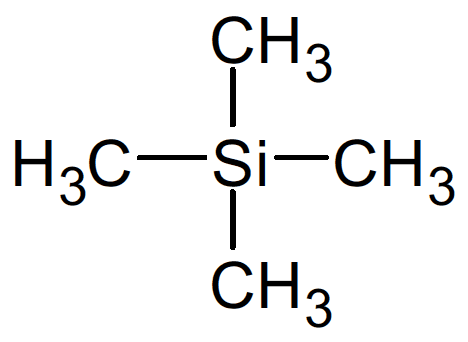
- Tetramethylsilane $\bf (TMS)$ is used as a standard as it has $12$ hydrogen atoms bonded in an identical atypical environmental close to a silicon atom. It gives one strong NMR signal away from signals produced by most organic compounds.
- The position of the $\rm NMR$ signal relative to the standard $\rm TMS$ is called the chemical shift of the proton. Hydrogen nuclei in particular environments have characteristic chemical shifts.
- The integrated trace indicates the relative number of hydrogen atoms in the different environments.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
