Although spectroscopic characterization techniques form the backbone of structural identification of compounds, typically no one technique results in a full structural identification of a molecule.
Nuclear magnetic resonance spectroscopy
- TMS is used as a standard reference in $\rm ^1H$ NMR spectroscopy as:
- The 12 protons are in the same chemical environment, so one strong, signal is produced $\rm (\delta = 0 ~ppm)$. All other chemical shifts are relative to this signal.
- It is chemically inert and can dissolve in most organic solvents.
- Silicon has a lower electronegativity value than carbon so TMS’s signal is generally separated from other proton signals in typical organic compounds.
- TMS is volatile so can be easily removed from a sample after measurement.
- Signals that appear as singlets in low resolution $\rm ^1H$ NMR spectra often appear as multiplets in high resolution spectra due to spin–spin coupling. If a proton has $n$ protons as nearest neighbours its NMR peak is split into $(n + 1)$ peaks.
- The splitting patterns can be deduced from Pascal’s triangle:
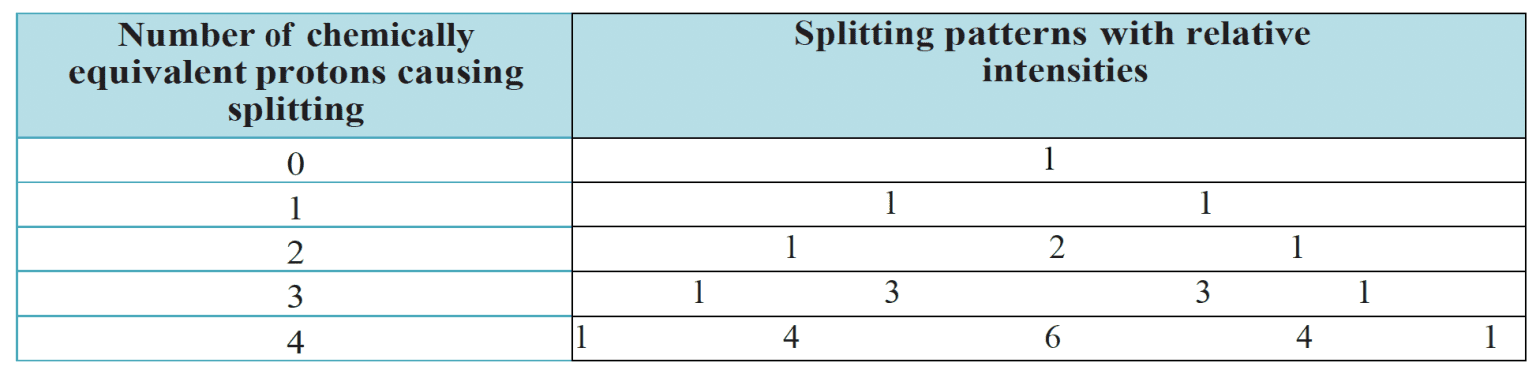
- When analysing high-resolution NMR spectra, the following additional points should be noted:
- Protons bonded to the same atom do not interact with one another as they are equivalent and behave as a group.
- Protons on non-adjacent carbon atoms do not generally interact with one another.
- The $\rm O—H$ single peak in ethanol does not split unless the sample is pure, as rapid exchange of the protons between ethanol molecules averages out the different possible spins.
X-ray diffraction
- X-rays are used to produce an electron density map of a crystalline solid. This can be related to the atoms which make up the molecule.
- The identity of the atoms can be determined from the pattern in electron densities which are related to an element’s electron configuration. Hydrogen atoms are not generally detected as they have only one electron.
- X-ray crystallography is used to identify all the bond lengths and bond angles of a crystalline compound from the arrangement of its atoms and therefore its geometrical arrangement in space.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
