Mole ratios in chemical equations can be used to calculate reacting ratios by mass and gas volume.
Reactions
- A limiting reactant is the reactant that is used up completely, an excess reactant is present in a reaction mixture in a quantity greater than needed to react.
- The limiting reactant determines the theoretical yield of product. The other reactants are in excess.
- The theoretical yield is the mass or amount of product produced according to the chemical equation, assuming 100% reaction of the limiting reagent.
- Percentage yield = (experimental yield/theoretical yield) × 100%
Gases
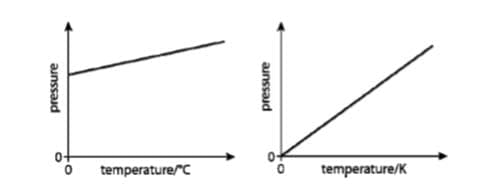
The kelvin is the $\rm SI$ unit of temperature: $\rm T (K) = T (°C) + 273$
- $\text{Units of volume:} \rm 1~dm^3 = 1 \times 10^{-3}~m^3$ $\rm = 1 \times 10^3~cm^3$
- For a fixed mass of an ideal gas at constant $\rm T: P = \dfrac{k_1}{V}$ $\rm (k_1~constant)$
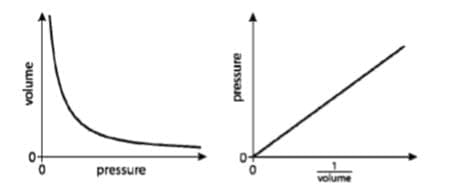
- For a fixed mass of an ideal gas at constant $\rm V: P = k_2T$
- The combined gas law: for a fixed mass of gas: $\rm\dfrac{P_1V_1}{T_1} = \dfrac{P_2V_2}{T_2}$
- Avogadro’s law states that equal volumes of gases measured at the same temperature and pressure contain equal numbers of molecules ($\rm V \propto n$, with $\rm T$ and $\rm p$ constant); the molar volume of an ideal gas is a constant at a specified temperature and pressure;
- The ideal gas equation: $\rm PV = nRT$
$\rm R = 8.31~J.K^{–1}~mol^{–1}$, $\rm T$ must be in $\rm K$. - Temperature (in $\rm K$) is a measure of the average kinetic energy of the particles. Particles have minimum kinetic energy at absolute zero $\rm (0~K)$.
- As kinetic energy $\rm = ½mv^2$ and all gases have the same kinetic energy at the same temperature, particles with smaller mass move faster.
- The molar volume of an ideal gas $\rm (V_{mol})$ is a constant at a specified temperature and pressure; Molar volume, $\rm V_{mol}$, of any gas at $\rm STP = 2.27 \times 10^{-2}~m^3~mol^{-1}$ $\rm = 22.7~dm^3~mol^{-1}$.
$\rm STP$ for gases is standard temperature $\rm(0 °C ~or~ 273~K)$ and pressure $\rm (100~ kPa)$.
$\text{Number of mol (n)} = \dfrac{\rm volume}{\text{molar volume}}$ $= \rm\dfrac{V}{V_{mol}}$ - The volume of the gases reacting and being formed in a chemical reaction is the same as the mole ratios in the chemical equation.
- $\rm Density = \dfrac{mass}{volume}$; $\rm \rho = \dfrac{m}{V}$
Solutions and Titrations
- A solution is a homogeneous mixture of a liquid (the solvent) with another substance (the solute). The solute can be solid, liquid, or gas but the solvent is generally a liquid.
- Concentration is the amount of solute in a known volume of solution. It can be expressed either in $\rm g~dm^{-3}$ or $\rm mol~dm^{-3}$. Concentration in $\rm mol~ dm^{−3}$ is often represented by square brackets around the substance:
$\rm [solute] (mol~dm^{-3}) = \dfrac{n_{solute} (mol)}{V_{solution} (dm^3)}$
$\rm n_{solute} = [solute] \times V_{solution} (dm^3)$
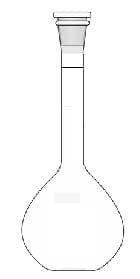
$\rm n_{solute} = [solute] \times \dfrac{V_{solution} (cm^3)}{1~000}$ - A standard solution is one with a known concentration of solute. It is prepared by adding solvent to a known mass of solute to form a solution of known volume. A Volumetric flask is a precise instrument for measuring the volume of solution.
 |
 |
|
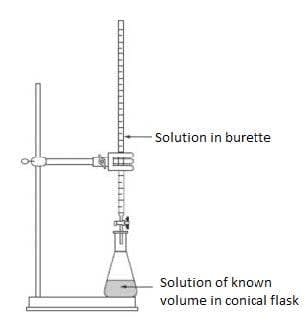
| A volumetric flask is used to prepare a standard solution. | A pipetted is used to measure a known volume precisely. | The set up for a titration. The volume of the solution in the conical flask is measured by a pipette. |
- Titration is a chemical technique in which one solution is used to analyse another solution to find its concentration or amount. A pipette is used to measure precisely the volume of one solution which is then added to a conical flask. Another solution is then added from a burette. This solution is added until the equivalence point of the reaction is determined. An indicator which changes colour at this equivalence point can be used to determine the end of reaction.
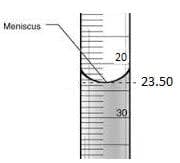
- When reading a burette, it is important to read the bottom of the meniscus. The burette reading is $23.50$.
- Acid- base and redox reactions are commonly investigated by titration.
- A back titration is an experimental procedure which typically consists of two consecutive acid-base titrations performed when an insoluble and slowly reacting reagent is treated with an excess of an acid or base. The excess acid or base is then titrated and neutralised with a primary standard. It can be used, for example, to analyse the amount of calcium carbonate in a shell or the amount of aspirin in a tablet.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
