The characterization of an acid depends on empirical evidence such as the production of gases in reactions with metals, the colour changes of indicators, or the release of heat in reactions with metal oxides and hydroxides.
- Characteristic reactions include:
$\rm acid + \text{reactive metal}$ $\rm \rightarrow salt + hydrogen$ - $\rm e.g.$ $\rm 2HCl(aq) + Mg(s)$ $\rm\rightarrow MgCl^2(aq) + H_2(g)$
$\text{acid + metal oxide}$ $\rm \rightarrow salt + water$
$\rm e.g.$ $\rm 2HCl(aq) + MgO(s)$ $\rm \rightarrow MgCl^2(aq) + H_2O(l)$
$\text{acid + metal hydroxide}$ $\rm \rightarrow salt + water$
$\rm e.g.$ $\rm 2HCl(aq) + Mg(OH)_2(s)$ $\rm \rightarrow MgCl_2(aq) + H_2O(l)$ - Soluble bases are called alkalis and produce hydroxide ions in aqueous solution.
$\rm e.g.$ $\rm MgO(s) + H_2O(l)$ $\rm \rightarrow Mg(OH)_2(aq)$
$\text{acid + metal carbonate}$ $\rm \rightarrow salt + water + \text{carbon dioxide}$
$\rm e.g.$ $\rm 2HCl(aq) + MgCO_3(s)$ $\rm \rightarrow MgCl_2(aq) + H_2O(l) + CO_2(g)$ - The reaction between an acid and a base to produce a salt is known as neutralization.
The ionic equation shows that reaction involves bond formation:
$\rm H^+_(aq) + OH^–(aq)$ $\rm \rightarrow H_2O(l)$
Neutralization reactions are exothermic. - The neutralisation of any strong acid with any strong base will produce the same net ionic equation and release the same amount of heat per mole of $\rm H^+(aq)$ or $\rm OH^–(aq)$ ions.
- Acid- base and redox reactions are commonly investigated by titration, a chemical technique in which one solution is used to analyse another solution to find its concentration or amount. An indicator which changes colour at the equivalence point can be used to determine the end of reaction.
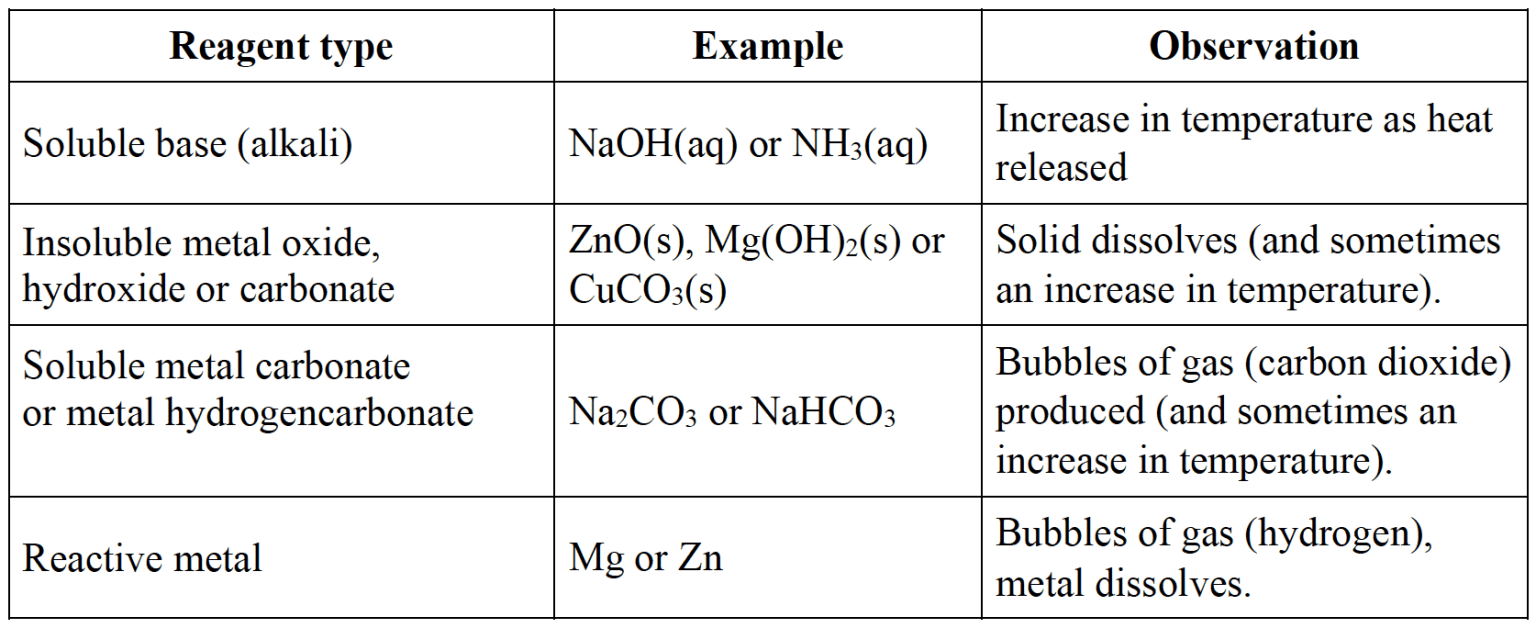
Some observations for acid reacitons.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
