pH curves can be investigated experimentally but are mathematically determined by the dissociation constants of the acid and base. An indicator with an appropriate end point can be used to determine the equivalence point of the reaction.
Buffers
- A buffer solution is resistant to changes in $\rm pH$ on the addition of a small amount of acid or base.
- Buffer solutions consist of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid.
- Buffers respond to added acid by reacting with the base in the buffer, and to added base by reacting with the acid in the buffer. These reactions cause a shift in the equilibria positions and by removing added $\rm H^+$ or $\rm OH^–$ keep the $\rm pH$ approximately constant.
- The $\rm pH$ of a buffer solution depends on:
- the $\rm K_a$ or $\rm K_b$ of the acid/base
- the ratio of the concentration of acid/base to salt.
- The $\rm pH$ of a buffer does not change with dilution.
- The $\rm pH$ of a buffer is temperature dependent.
- The buffer capacity refers to the amount of acid or base that can be added before the $\rm pH$ changes dramatically. This is reduced with dilution of the buffer.
pH Curves in Titrations
- The $\rm pH$ of a salt solution is determined by the hydrolysis of its ions in aqueous solution. This depends on the relative strength of the parent acid and base. The $\rm pH =7$ when a strong acid is neutralised by a strong base ($\rm e.g.$; $\rm NaCl$ is formed from $\rm HCl$ and $\rm NaOH$) but this is not the case for other titrations.
- Cation hydrolysis: the conjugate from weak bases causes the $\rm pH$ of the solution to be acidic at the equivalence point:
$\rm eg: NH^+_4(aq) + H_2O(l)$ $\rightleftharpoons \rm NH_3(aq) + H_3O^+(aq)$ - Anion hydrolysis: the conjugate from weak acids, causes the $\rm pH$ of the solution to be alkaline $\rm e.g.$ $\rm CH_3COO^- + H_2O(l)$ $\rightleftharpoons \rm CH_3COOH(aq) + OH^-(aq)$
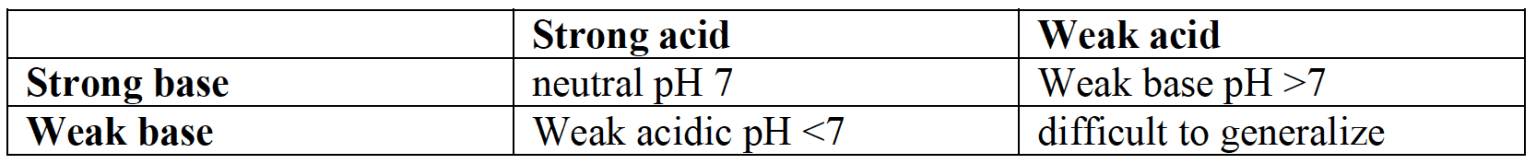
- The equivalence point occurs when equal stoichiometrically amounts of acid and base have reacted together, so the solution contains a salt and water only. The $\rm pH$ of the salt formed depends on the relative strength of acid and base that reacted together as shown in the table.
pH of salt solutions
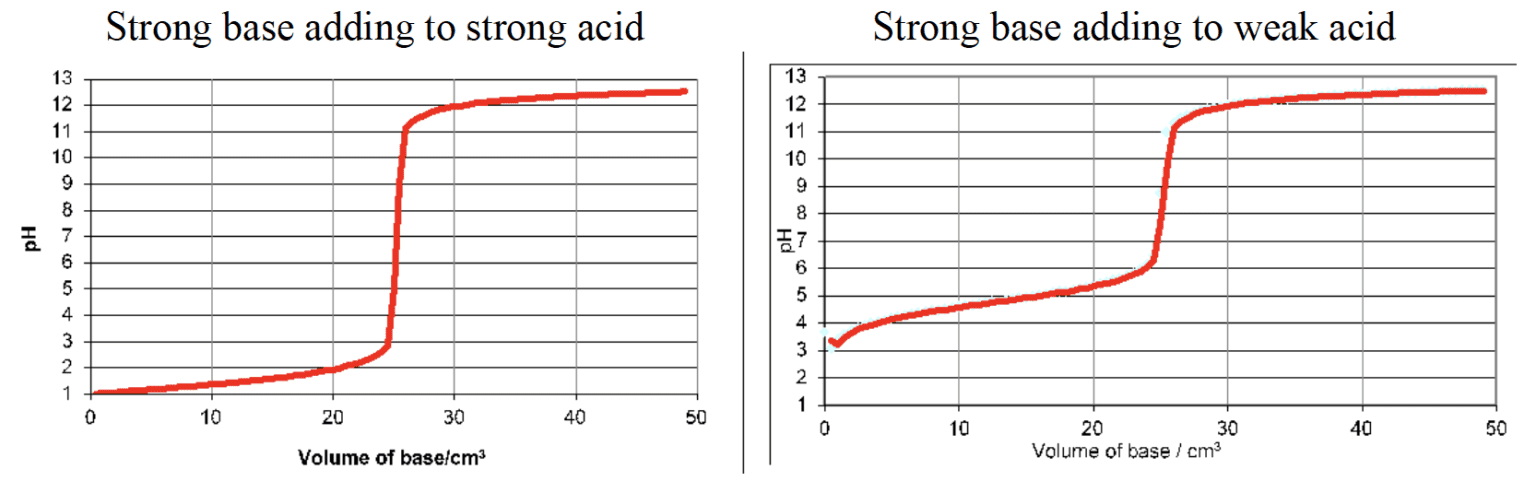
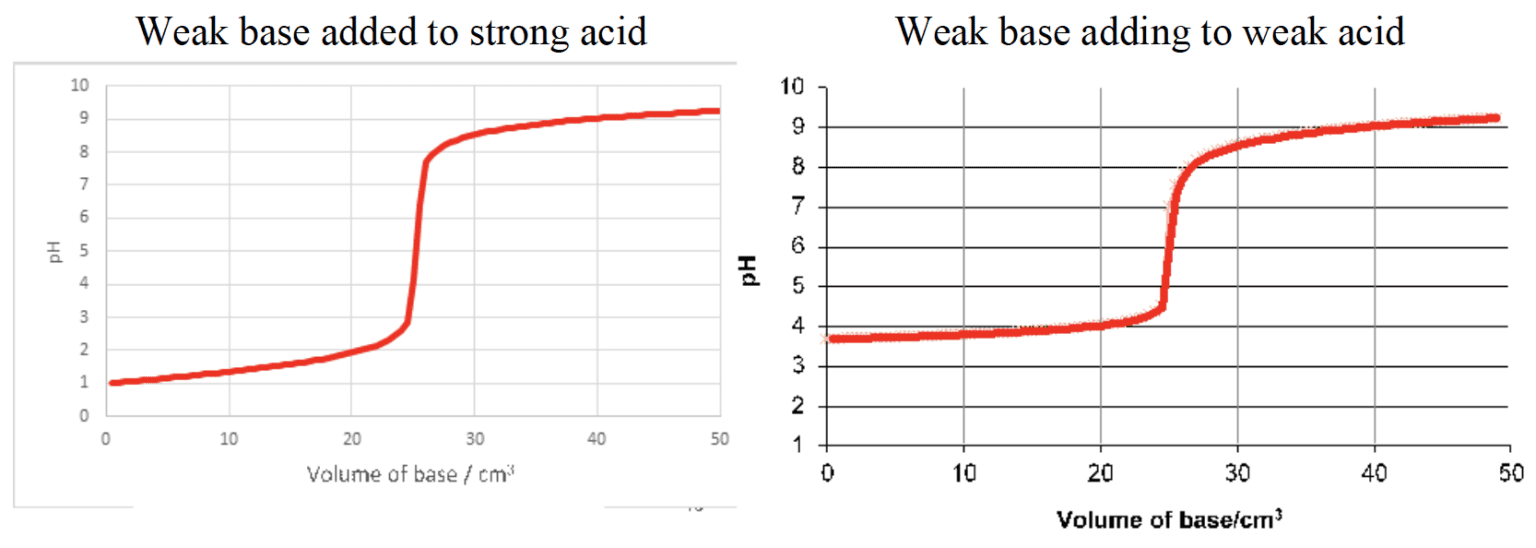
- $\rm pH$ curves show the change in $\rm pH$ as a base is added to an acid, or vice versa, in a titration reaction.
- The intercept with the $\rm pH$ axis shows the initial $\rm pH$ of the acid or base.
- The buffer region on the $\rm pH$ curve represents the region where small additions of acid or base result in little or no change in $\rm pH$.
- The $\rm pH$ at equivalence depends on the relative strengths of the acid and base reacted together.
- There is a large change in the $\rm pH$ at equivalence point, known as the point of inflection.
- $\rm pH$ at half-equivalence point $\rm = pK_a$ of acid.
Indicators
- An indicator is a weak acid or a weak base, in which the acid or base and its conjugate have different colours.
- The $\rm pH$ at which an indicator changes colour is known as its end-point. This occurs when the concentration of dissociated and undissociated forms are equal: $\rm [HIn] = [In^-]$
- The end-point of an indicator is when $\rm pH =$ its $\rm pK_a$ or $\rm pK_b$.
- An indicator is appropriate for use in a titration when its end-point ($\rm pK_a/pK_b$ value) falls within the range of the $\rm pH$ of the equivalence point.
- For an indicator to be suitable in a titration the $\rm pH$ of its end point must coincide with the $\rm pH$ at the equivalence point.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
