Elements show trends in chemical and physical properties down groups and across periods.
Electron configuration and atomic and ionic radii
- The chemical and physical properties of elements arranged in order of increasing atomic numbers display periodicity. Periodicity is the regular repetition of properties of the elements arising from patterns in their electron configuration.
- Outer electron are shielded from the nucleus by the inner electrons. The effective nuclear charge is a measure of the nuclear charge they experience due to the presence of these inner electrons. It can be approximated as being equal to the group number for the outer electrons.
- As a group is descended the nuclear charge increases but the effective nuclear charge stays approximately constant. The number of occupied energy levels increase down a group which results in an increase in the atomic and ionic radii.
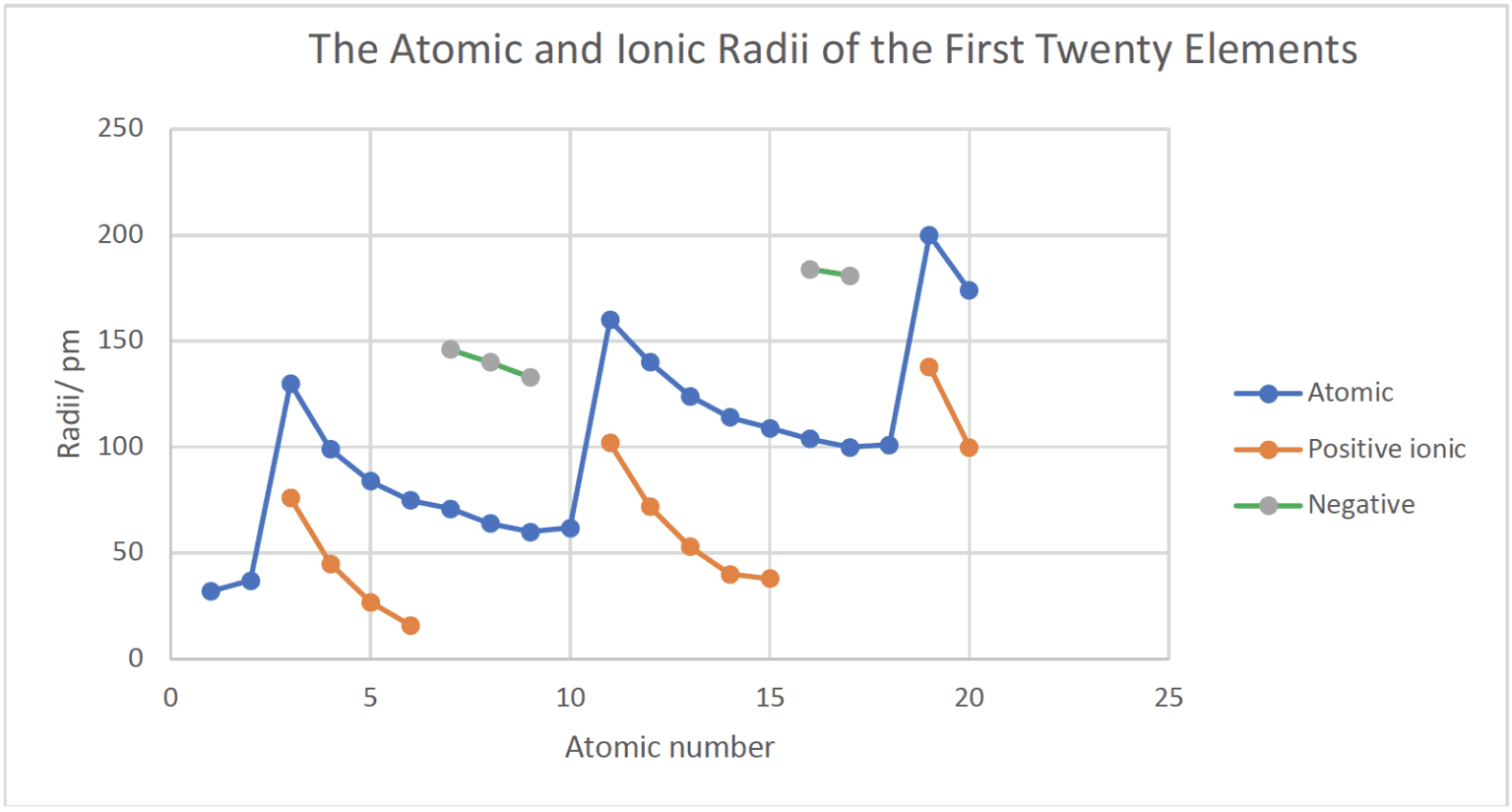
- Across a period the atomic radii decrease as the nuclear charge (and effective nuclear charge) increases and electrons are added to the same outer energy level. The electrostatic attraction between the outer electrons and nucleus increases.
- Patterns in ionic radii in period 3 are more complex:
- Positive ions are smaller than their parent atoms as the formation of positive ions involves the loss of electrons from the outer energy level.
- Negative ions are larger than their parent atoms as their formation involves the addition of electrons into the outer energy level. The increased electron repulsion between the electrons in the outer energy level increases the radius of the outer energy level.
- The ionic radii decrease from Groups $1$ to $4$ for the positive ions. The ions $\rm Na^+$, $\rm Mg^{2+}$, $\rm Al^{3+}$ and $\rm Si^{4+}$ are isoelectronic and have the same electron configuration $\rm 1s^22s^22p^6$. The decrease in ionic radius is due to the increase in nuclear charge with atomic number across the period. This increases the electrostatic attraction between the nucleus and the outer electrons.
- The ionic radii decrease from Groups $4$ to $7$ for the negative ions. The ions $\rm Si^{4-}$, $\rm P^{3-}$, $\rm S^{2-}$ and $\rm Cl^-$ are isoelectronic and have the same electron configuration arrangement $\rm 1s^22s^22p^63s^23p^6$. The decrease in ionic radius is due to the increase in nuclear charge across the period.
- Positive ions are smaller than parent atoms; The positive ions have only two occupied electron energy levels and the latter have three. Negative ions are larger than parent atoms due to an increase in electron repulsion between the electrons in the outer energy level. $\rm R_{cation} < R_{atom} < R_{anion}$
Patterns in melting points
- Melting points depend on the bonding and structure of a material.
The Melting Points of the First 20 Elements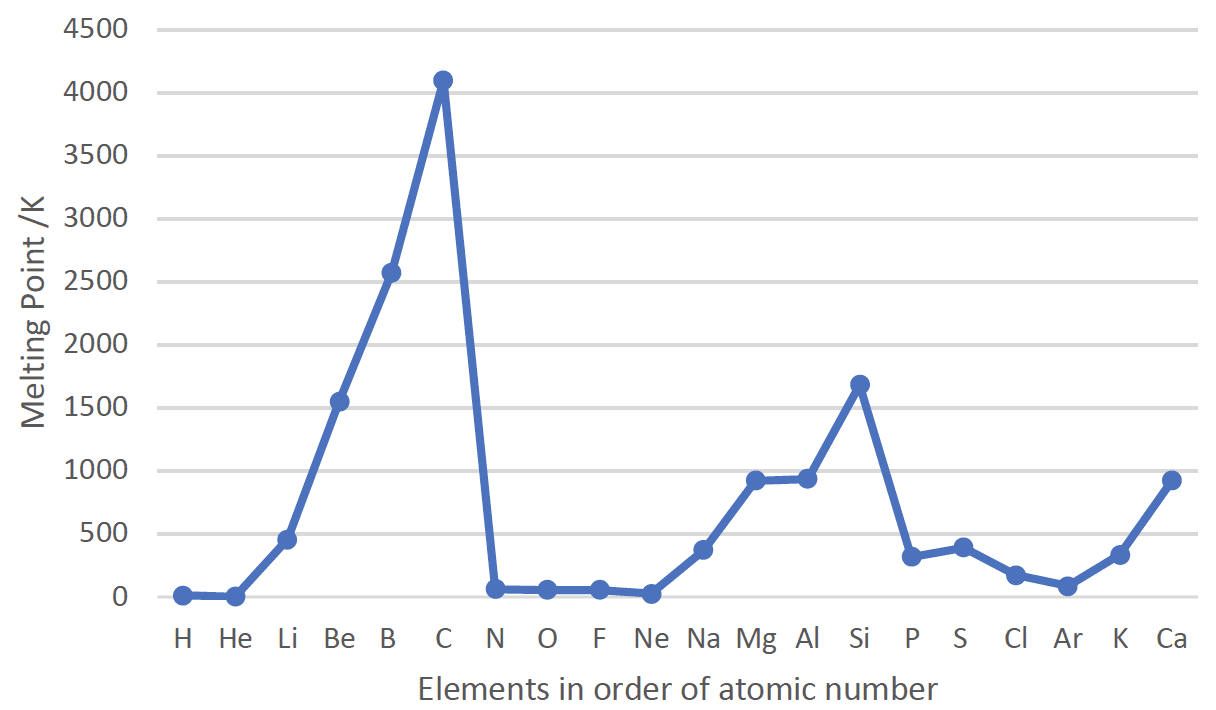
- The melting points decrease down group $1$ as there is a decrease in the strength of the metallic bonding. The increase in ionic radii reduces the force of the electrostatic attraction between the $\rm M^+$ ions and the delocalised electrons.
- The melting points increase down group $17$ as there is an increase in the strength of the dispersion intermolecular forces with increasing number of electrons.
- Melting points increase across period $2$ and $2$ to a maximum in group $14$ for carbon and silicon and then decreases to a minimum for the noble gases.
Patterns in ionization energy and electronegativity
- The first ionization energy $\bf (IE_1)$ of an element is the minimum energy required to form a mole of singly charged positive ions $\rm (M^+)$ by removing an electron from each atom $\rm (M)$ in the gaseous state: $\rm M(g) \rightarrow \rm M^+ (g) + e^–$ (units: $\rm kJ~mol^{–1})$.
- Electronegativity is a measure of how strongly the atom attracts the electrons in a covalent bond.
The Electronegativity of the first twenty elements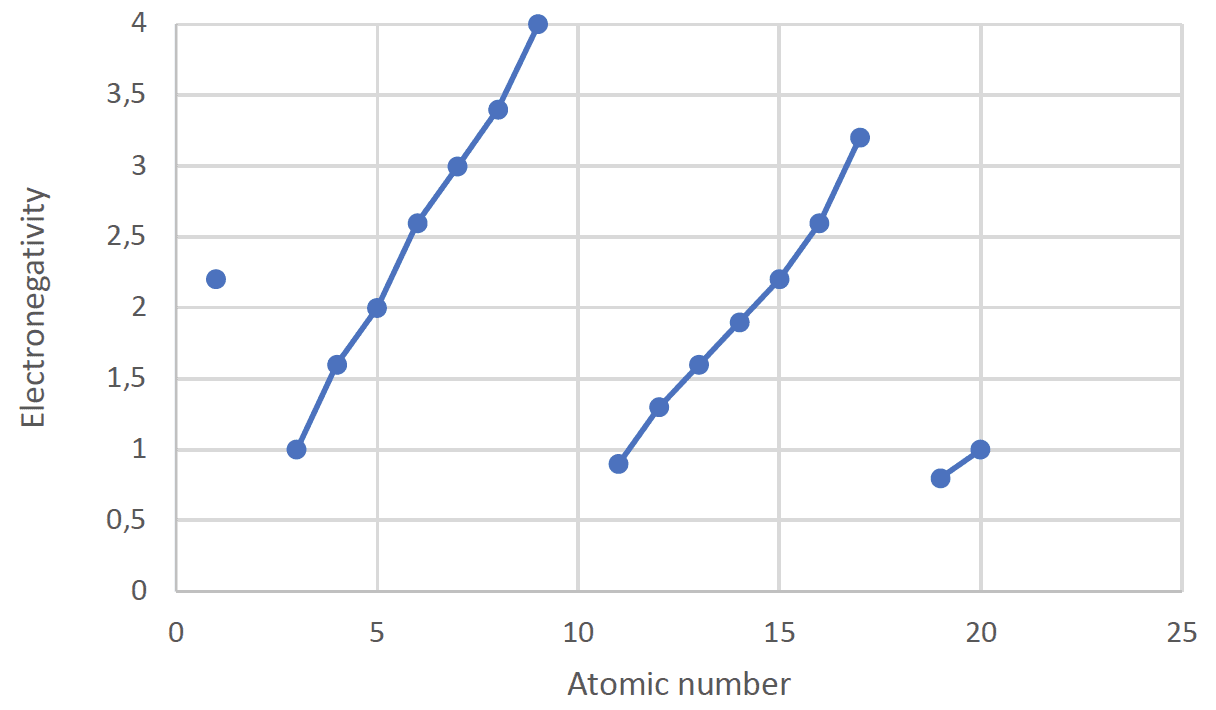
- The first ionization energies and electronegativities increase across a period as the nuclear charge increases. The electrons are added to the same outer shell resulting in an increase in the attraction between the outer electrons and nucleus increases.
- As a group is descended both first ionization energies and electronegativities decrease due to the increased distance between the nucleus and the outer energy level which reduces the force of attraction between the nucleus and the outer electrons.
- The noble gases are not assigned electronegativities as they do not readily form bonds with other elements.
- The electronegativities of diagonal elements remain approximately the same as both the group and period number increase. Boron and aluminium for example both have electronegativities of $1.6$.
- The electronegativity of $\rm H$ is the same as that of $\rm P$.
- There are regular discontinuities in the trend of first ionization energies across a period, due to the existence of sub-levels within the main energy levels.
- Metals have low ionization energies and electronegativities. Non-metals have high ionization enemies and electronegativities.
- The Electron affinity is the energy change when one mole of electrons are added to one mole of gaseous atoms to form one mole of gaseous ions: $\rm X(g) + e^- \rightarrow X^-(g)$.
- First electron affinities become more exothermic along a period due to the increase in nuclear charge.
- The chemical properties of the elements are generally due to the number of electrons in the outer energy level of their atoms.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA