The arrangement of elements in the Periodic Table helps to predict their electron configuration.
Periods and Groups
- The Periodic Table arranges elements according to their increasing atomic number / proton number. It can be used to correlate both the physical and chemical properties of elements.
- The horizontal rows are called periods and vertical column are called groups.
- The period number $\rm [n]$ is the outer energy level that is occupied by electrons.
- Elements in the same period have outer electrons in the same energy level.
- The groups are numbered from $1$ to $18$.
- Elements: in the same group have the same number of outer electrons and have similar chemical properties.
- The Periodic Table is arranged it $4$ blocks $\rm -s,$ $\rm p,$ $\rm d$ and $\rm f-$ which correspond to the highest sub-level occupied by electrons.
- The position of an element is related to the electron configuration of the atoms. Potassium, for example is in Period $4$ as it has four occupied energy levels and is in Group $1$ as there is one electron in its outer energy level. It is in the s block as this outer electron in is in an $\rm s$ orbital.
Metals and non-metals
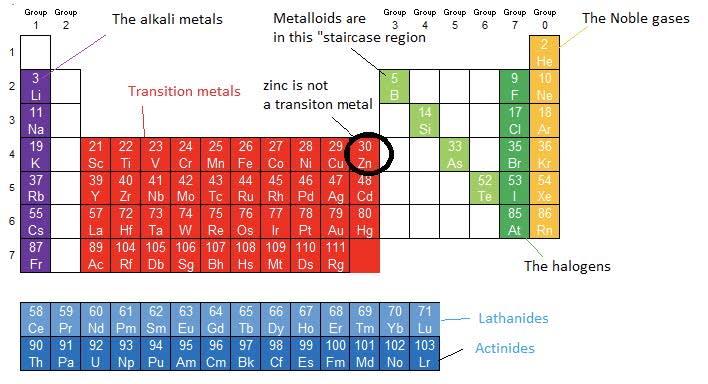
- Metals are found on the left of the Periodic Table and non-metals on the right side. Metalloids form a diagonal staircase between the metals and non-metals.
- The alkali meals are in Group $1$.
- The halogens are in Group $17$.
- The Noble gases are in Group $18$.
- The transition metals are in a large rectangular section - the $\rm d$-block elements in the middle of the Periodic Table. They are elements with an incomplete $\rm d$-sublevel or form ions with an incomplete $\rm d$-sublevel. Although in the $\rm d$-block zinc is not considered a transition element as it has a full $\rm 3d$ sublevel, with a condensed electron configuration $\rm [Ar]3d^{10}4s^2$.
Lanthanides and actinides are metals in the first and second row of the $\rm f$ block which forms an island off the main body of the periodic table.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
