Redox (reduction–oxidation) reactions play a key role in many chemical and biochemical processes.
Oxidation and reduction
- Oxidation and reduction can be defined in terms of oxygen, hydrogen and electrons loss or gain, or change in oxidation state:
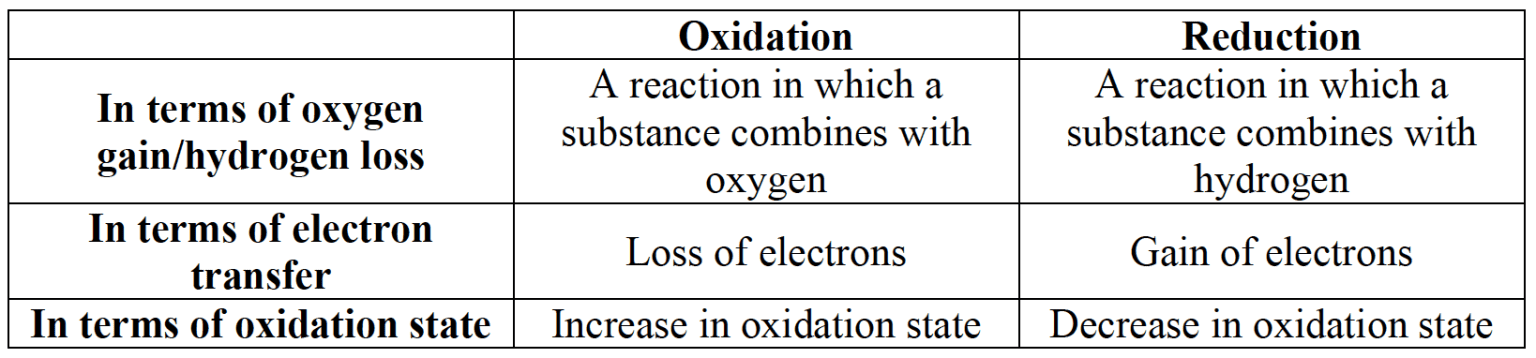
- The oxidation state is a measure of the apparent charge of an atom in a free element, a molecule or an ion.
- Oxidizing agents oxidize other species and are themselves reduced.
- Reducing agents reduce other species and are themselves oxidized.
- Transition metals and most non-metals in the p-block have variable oxidation states.
- Oxidation numbers are used to represent the oxidation states of elements in names of compounds, using Roman numerals.
Worked Example
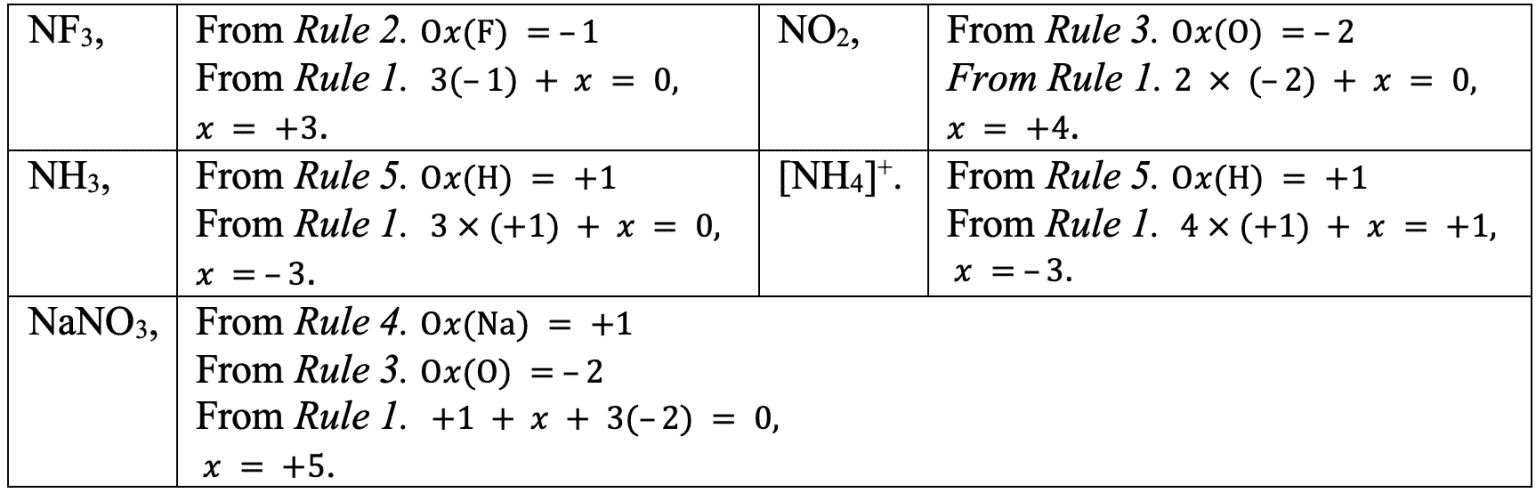
Deduce the oxidation state of nitrogen in the following.
![]()
Solution
There are some rules for applying oxidation states:
- The sum of all oxidation states in a species is equal to the total charge.
- The oxidation state of fluorine in its compounds is always $–1$.
- The oxidation state of oxygen in a compound is usually $–2$. However the oxidation state of oxygen in peroxides $\rm (eg$ $\rm H_2O_2)$ is $–1$. When the oxygen is bonded to fluorine, the oxidation state is $+1$.
- Group 1 metals in their compounds have an oxidation state of $+1$; Group 2 metals in their compounds have an oxidation number of $+2$.
- The oxidation state of hydrogen in a compound is $+1$ unless in a Group 1 or 2 hydride when it is $–1$.
- Chlorine, bromine, and iodine usually have an oxidation state of $–1$, unless they’re in combination with an oxygen or fluorine.
Let $x$ be equal to the oxidation state of nitrogen.
Reactivity
- Metal elements react by losing their outer electrons. They are oxidised and act as reducing agents.
- The activity series ranks metals according to the ease with which they undergo oxidation.
- The more reactive a metal, the stronger it is as a reducing agent.
- Non-metallic elements react by gaining electrons. They are reduced and act as oxidising agents.
- The more reactive a non-metal, the stronger it is as an oxidizing agent.
- More reactive metals are able to reduce the ions of less reactive metals in displacement reactions.
- More reactive non-metals are able to oxidize the ions of less reactive non-metals in displacement reactions.
- Metals more reactive than hydrogen produce hydrogen gas when added to dilute acids.
- Metals less reactive than carbon can be extracted by reducing the metal oxide by the carbon.
![]()
Half-equations
- Half-equations show the electrons lost/gained in oxidation/reduction reactions and can be used as a step in balancing a redox equation.
- Redox titrations are used to determine concentrations of solutions by finding the equivalence point when two reactants have reacted stoichiometrically, by transferring electrons from the reducing agent to the oxidizing agent.
- The Winkler method uses a redox titration to measure the dissolved oxygen content of water.
The oxygen oxidises $\rm 2Mn^{2+}(aq)$:
$\rm 2Mn^{2+}(aq) + 4OH^-(aq) + O_2(aq)$ $\rightarrow\rm 2MnO_2(s) + 2H_2O(l)$
The $\rm MnO_2(s)$ produced then oxidises iodide ions to iodine:
$\rm MnO_2(s) + 2I^-(aq) + 4H^+(aq)$ $\rm \rightarrow Mn^{2+}(aq) + I_2(aq) + 2H_2O(l)$
The iodine is then titrated with thiosulfate:
$\rm I_2(aq) + 2S_2O^{2-}_3(aq)$ $\rm \rightarrow 2I^-(aq) + S_4O^{2-}_6(aq)$
The concentration of $\rm O_2(aq)$ can be determined by the volume of thiosulfate solution.
Worked Example
Nitric acid reacts with silver in a redox reaction.
$\rm Ag(s) + NO^–_3(aq) + \underline{\quad}$ $\rm \rightarrow Ag^+(aq) + NO(g) + \underline{\quad}$
Deduce the complete balanced equation for the reaction.
Solution
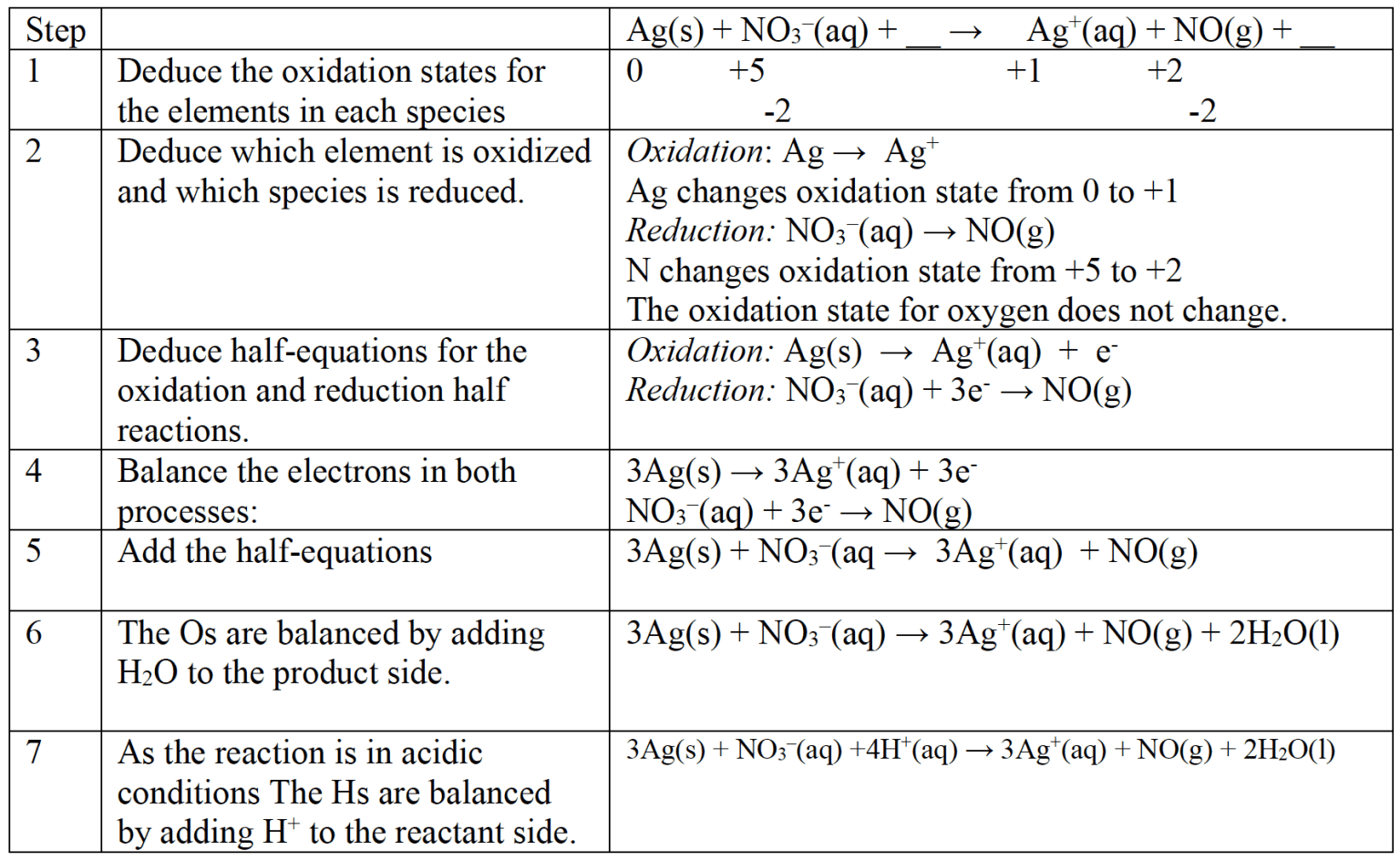
Note the atoms of each element and the charges are balanced ($3+$ on both sides).
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
