Energy can be released in nuclear reactions as mass is converted to energy.
- A nucleus with $\rm Z$ protons and $\rm N$ neutrons has a mass that is less than the sum of the mass of the protons and neutrons. The mass defect $(\Delta_{\text {mass defect }})$ is this difference.
$\rm\Delta mass_{defect}=\mathcal n_{proton}\times \mathcal m_{proton}$ $+$ $\rm \mathcal n_{neutron} \times \mathcal m_{neutron} - M_{nucleus}$
$\rm \Delta mass_{defect} = Z \times \mathcal m_{proton} + (A - Z) \mathcal m_{neutron} - M_{nucleus}$ - The mass is reduced as energy is given out as the nucleus is formed. The binding energy is the amount of energy released. It is also the minimum energy needed to reverse this process and separate a nucleus into its individual nucleons.
$$
\Delta \rm E_{BE} = \Delta mass_{defect} ~\mathcal c^{2}
$$ - A graph of the binding energy per nucleon against nucleon number $A$ shows a peak around the nuclei $\mathrm{Fe}-56 / \mathrm{Ni}-62$. These are the most stable nuclei.
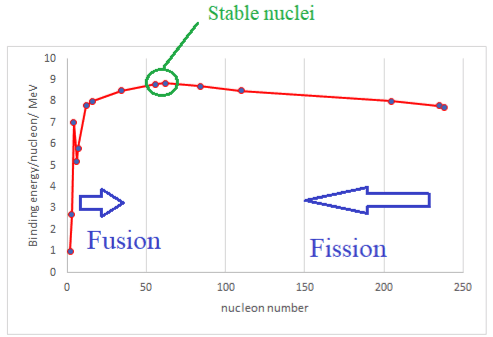
- Most nuclei have approximately the same binding energy per nucleon of $\rm 8.5~ MeV$. This similarity in binding energy per nucleon is due to the short range of the strong nuclear force: in a large nucleus any one nucleon is surrounded by the same number of nucleons and so it takes the same energy to remove it.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
