The properties of ideal gases allow scientists to make predictions of the behaviour of real gases.
Boyle’s law
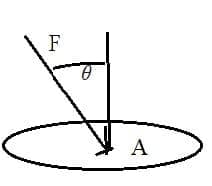
- Pressure $(\mathrm{P})=$ force $\rm (F)$ per unit area $\rm (A)$
$\rm P=F / A$
The Unit: $\mathrm{N} \mathrm{m}^{-2}$ or pascal $(\mathrm{Pa})$ - As gas pressure acts equally in all directions there is no resultant pressure in any direction: it is a scalar property.
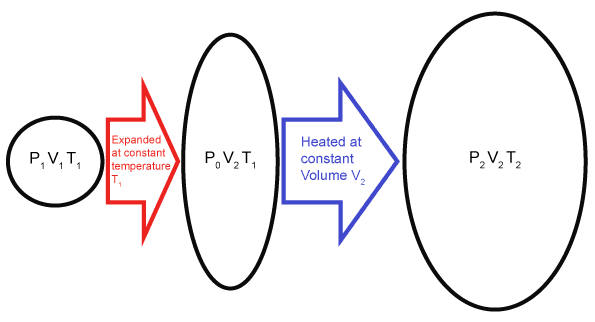
- The pressure of a fixed mass of gas at constant temperature is inversely proportional
to its volume:
$\rm P \propto 1 / V$
$\rm P= \mathcal k / V$ where $k$ is a constant that depends on the amount and temperature of the gas
$\rm P V=\cal k$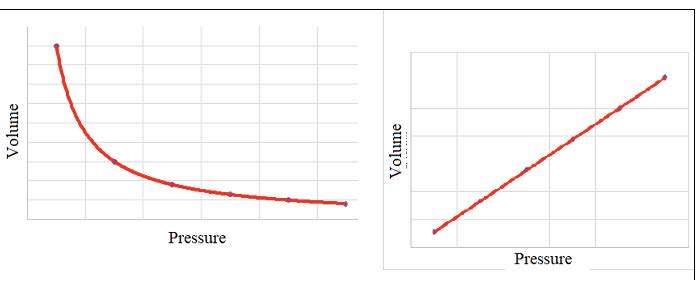
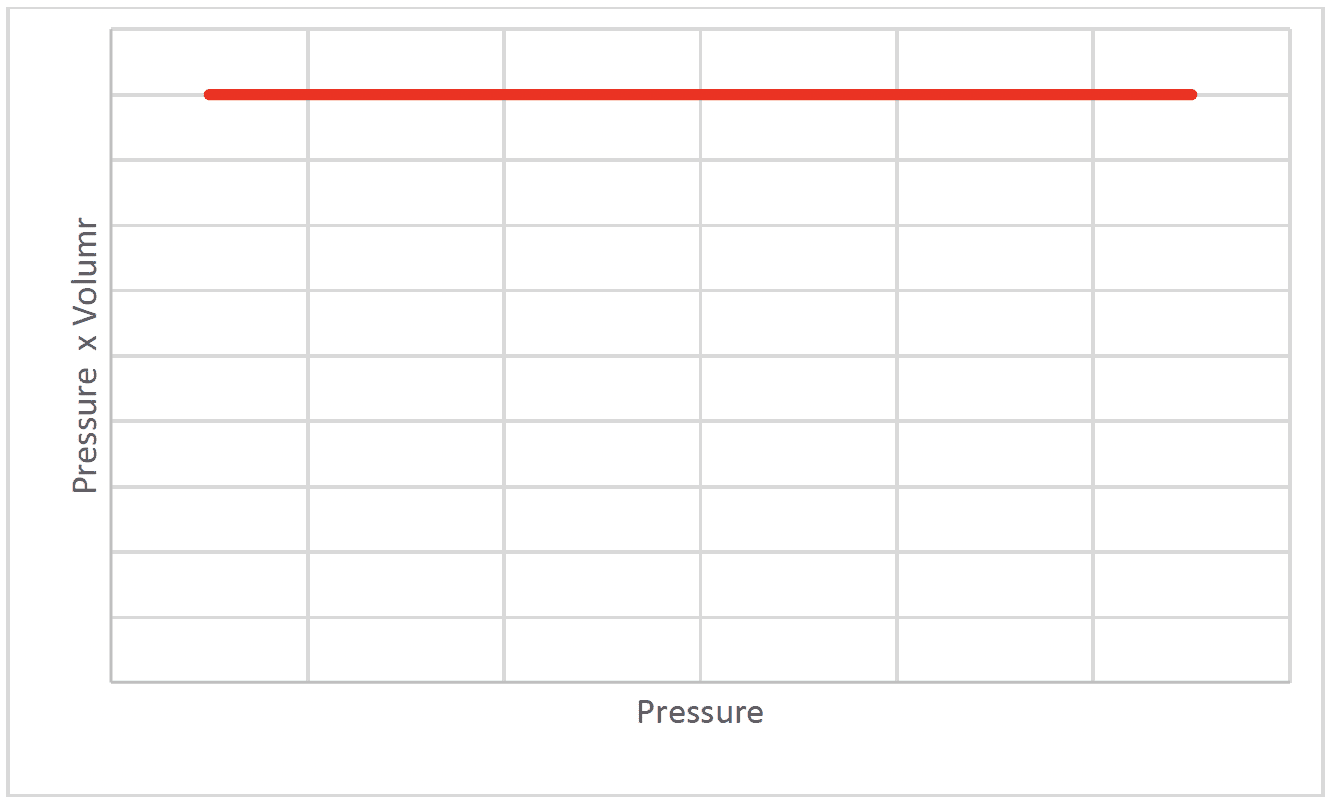
If the volume of a gas decreases the particles will collide with the walls more often and the pressure increases.
Pressure law
- The pressure of a fixed mass of gas with constant volume is directly proportional to its temperature in kelvin.
- Increasing temperature increases the average $\mathrm E_k$ of the molecules. Molecules collide with the walls more frequently and with more momentum.
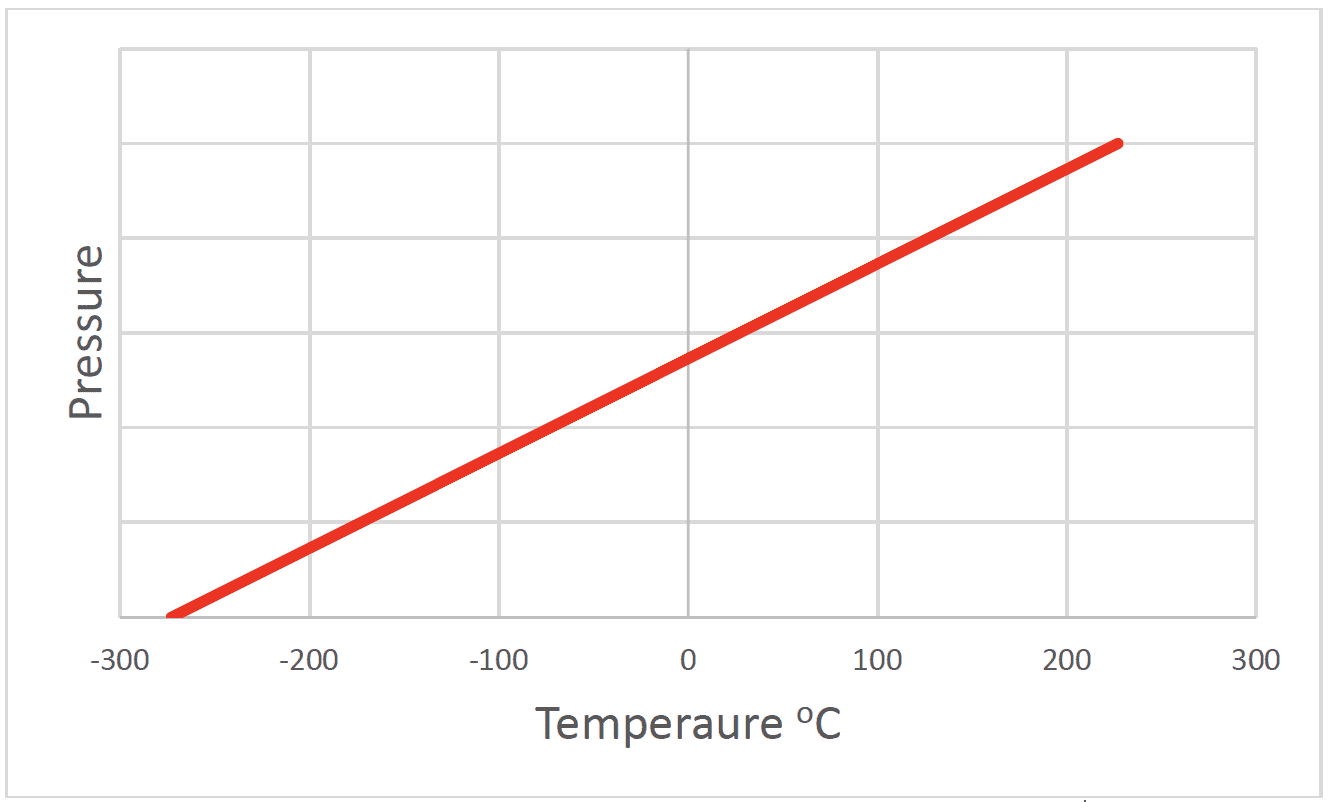
The pressure of a gas is $0$ at $\rm 273°C$. This is absolute zero $\rm 0~K$.
Charles’ law
- The volume of a fixed mass of gas at a constant pressure is directly proportional to its temperature in kelvin.
$\mathbf{V} \propto \mathbf{T}(\mathbf{K})$
An increase in temperature causes the particles to move faster. The pressure is constant if the volume increases, and the particles collide less frequently.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA