Metallic bonds involve a lattice of cations with delocalized electrons.
Metallic bonding involves delocalised electrons
- Metal atoms are held together by the electrostatic attraction between a lattice of positive ions and delocalized electrons.
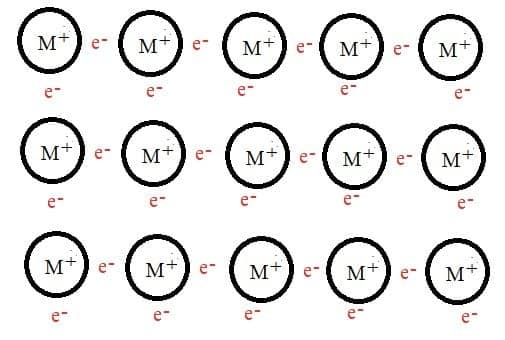
- The strength of the metallic bond increases with increasing charge on the cation and decreases with the increasing radius of the ion. The metallic bonding in transition metals is generally strong due as the electrons in d-orbitals can become delocalised throughout the structure.
Physical properties and alloys
- The properties of metals – electrical and thermal conductivity, malleability, ductility − are a result of the delocalized electrons, which allow the bonds to be non-directional. Atoms can slide across each other without changing the attraction between the ions and the delocalised electrons.
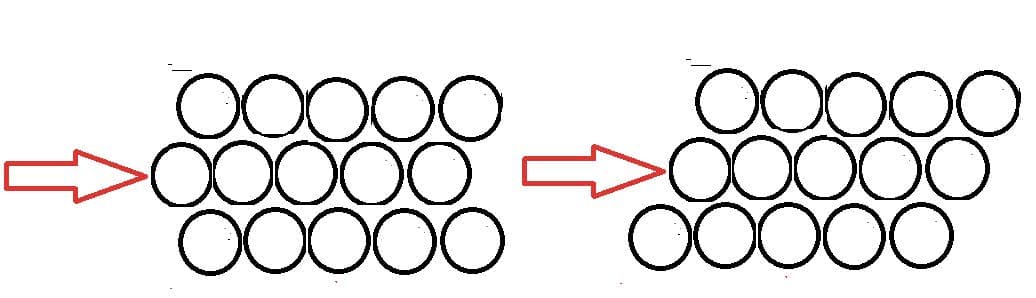
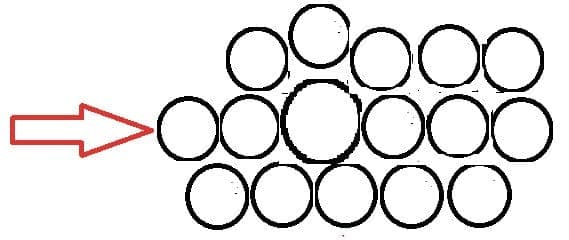
- Alloys are generally mixtures of metals. Steel is a mixture of iron and carbon with other metals.
- The presence of atoms of difference size makes the structure less regular. This makes it more difficult for layers of atoms to slide across each other increasing the structure stronger.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
