The enthalpy changes from chemical reactions can be calculated from their effect on the temperature of their surroundings
Enthalpy of reaction
- Heat is a form of energy transfer.
- Energetics deals with heat changes in chemical reactions or physical changes of state ($\rm e.g. ~(l)$ to $\rm (g)$ etc.).
- Temperature is a measure of average kinetic energy of the particles.
- Total energy is conserved in chemical reactions.
- Enthalpy is the amount of heat energy contained in a substance. It is stored in the chemical bonds as potential energy. When substances react, the difference in the enthalpy between the reactants and products (at constant pressure) results in a heat change which can be measured.
- The reaction mixture is called the system and anything outside the system is called the surroundings : $\text{the universe = system + the surroundings}$
- Thermochemical equations give the balanced equation with the enthalpy change $\rm (\Delta H^{\theta})$ in $\rm kJ~mol^{-1}$.
$\rm e.g.$ $\rm H_2(g) + ½O_2(g) \rightarrow H_2O(l)$;
$\rm \Delta H^{\theta} = -286~kJ~mol^{-1}$
$\rm H_2(g) + ½O_2(g) \rightarrow H_2O(g)$;
$\rm \Delta H^{\theta} = -242~kJ~mol^{-1}$
State symbols must be shown as $\rm \Delta H^{\theta}$ depends on the state of the reactants or products.
- Chemical reactions that involve transfer of heat between the system and the surroundings are described as exothermic or endothermic reactions.
- In exothermic reactions heat is released to the surroundings. $\rm \Delta H^{\theta}$ is negative and the temperature of the reaction mixture increases as the chemicals give out heat.
- In endothermic reactions heat is absorbed from the surroundings. $\rm \Delta H^{\theta}$ is positive and the temperature of the reaction mixture decreases as the chemicals absorb heat.
- Enthalpy changes can be shown on an energy level diagram:
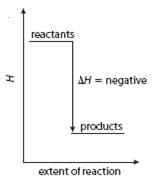
An exothermic reaction: The products are more stable than the reactants as they have a lower enthalpy.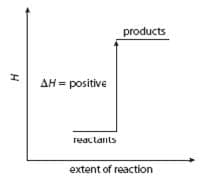
An endothermic reaction: The products are less stable than the reactants as they have a higher enthalpy. - The standard state of an element or compound is its most stable state under standard conditions (pressure $\rm 100~kPa$, temperature $\rm 298~K$).
- The standard enthalpy change $(\rm \Delta H^{\theta})$ is the heat energy transferred under standard conditions (pressure $\rm 100~kPa$, temperature $\rm 298~K$, concentration $\rm 1~mol~dm^{-3}$). Only enthalpy changes $(\rm \Delta H^{\theta})$ can be measured, not absolute enthalpies $\rm (H)$ for the initial or final state of a system.
- The standard enthalpy change of combustion $\rm (\Delta H^o_{comb})$ is the enthalpy change for the complete combustion of one mole of a substance in its standard state in excess oxygen under standard conditions. Most combustion reactions are exothermic.
- The standard enthalpy change of formation $\rm (\Delta H^o_{form})$ is the enthalpy change when mole of the substance is formed from its elements in their standard states under standard conditions. For a pure element in its standard state, $\rm \Delta H^o_{form} = 0$;
- The enthalpy of neutralization $\rm (\Delta H^o_{neut})$ is the enthalpy change when one mol of $\rm H^+(aq)$ reacts with one mol of $\rm OH^-(aq)$ ions. The reaction is exothermic as bond formation takes place:
$\rm H^+(aq) + OH^-(aq) \rightarrow H_2O(l)$.
Calorimetry
- Calorimetry is the technique of measuring heat changes in physical processes and chemical reactions.
- Heat changes can be calculated from the temperature changes:
$\text{heat change (q)} = \text{mass (m)}$ $\times$ $\text{specific heat capacity (c)}$ $\times$ $\text{temperature change}$ $\rm (\Delta T)$ - The specific heat capacity is the amount of heat energy required to raise the temperature of unit mass $\rm (e.g.~1~kg$ or $\rm 1~g)$ of a substance, by $\rm 1°C$ or $\rm 1~K$.
- $\rm (\Delta H^o_{comb})$ and $\rm (\Delta H^o_{reaction})$ for reactions in aqueous solution can be calculated if it is assumed that all the heat produced/absorbed enters/leaves the water.
$\rm (\Delta H^o_{comb}) = -\mathcal m_{H_2O} \times \mathcal c_{H_2O} \times \dfrac{\Delta T_{H_2O}}{\mathcal n_{fuel}}$
The experiment is performed with a calorimeter which is a good conductor. This allows heat from the flame to pass to the water.$\rm (\Delta H^o_{reaction}) = -\mathcal m_{H_2O} \times \mathcal c_{H_2O}$ $\times$ $\rm \dfrac{\Delta T_{H_2O}}{\mathcal n_{\text{limiting reagent}}}$
The experiment is performed with a calorimeter which is an insulator of heat, which reduces heat losses from the system.
- If a calorimeter absorbs heat: $\rm Q = (\mathcal m_{H_2O} \times \mathcal c_{H_2O} \times \Delta T_{H_2O})$ $+$ $\rm (\mathcal m_{calor} \times \mathcal c_{calor} \times \Delta T_{calor})$
- Heat loss and incomplete combustion can lead to systematic errors in experimental results.
Worked Example
The enthalpy of combustion of $\rm propan–1–ol$ can be determined experimentally.
A student collected the following data in an experiment.
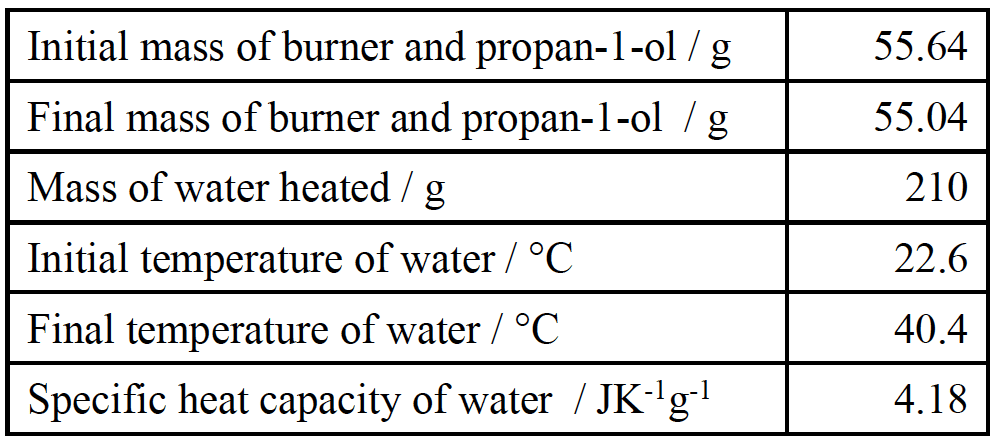
- Calculate the amount, in mol, of $\rm propan–1–ol$ burned.
$\rm\color{cornflowerblue}{\mathcal m(propan–1–ol)}$ $ \color{cornflowerblue}{= (55.64 – 55.04)}$ $\rm \color{cornflowerblue}{= 0.60 (g)}$;
$\rm\color{cornflowerblue}{\mathcal n(propan-1-ol)}$ $\color{cornflowerblue}{= \dfrac{0.60}{60.11} = 0.00998}$ - Calculate the heat absorbed, in $\rm kJ$, by the water.
$\color{cornflowerblue}{(ii)}$ $\rm\color{cornflowerblue}{\Delta T = (40.4 – 22.6) = 17.8~(K)}$
$\color{cornflowerblue}{\rm q = (mc∆T =) 210 × 4.18 × 17.8 (J)}$
$\color{cornflowerblue}{\rm 15.62 (kJ)}$ - Determine the enthalpy change, in $\rm kJ~mol^{-1}$, for the combustion of one mole of $\rm propan–1–ol$
$\color{cornflowerblue}{\rm =\dfrac{15.62}{0.00998}}$
$\color{cornflowerblue}{\rm - 1565~(kJ ~mol^{-1)}}$ - Suggest why this value differs from the literature value of $\rm −2021~kJ~ mol^{-1}$.
not all heat produced transferred to water as heat is lost to surroundings
incomplete combustion with carbon and carbon monoxide being formed instead of carbon dioxide also leads to a less exothermic reaction.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
