Ionic compounds consist of ions held together in lattice structures by ionic bonds.
Ions
- An ion is a charged particle formed when atoms lose ort gain electrons
- Positive ions are sometimes called cations and negative ions anions or oxyanions if the anion contains oxygen.
- The number of charges on an ion is equal to the number of electrons lost (positive ion) or gained (negative ion) by an atom.
- Metals lose electrons to form positive ions (cations); non-metals gain electrons to form negative ions (anions).
- The charge on an ion can usually be predicted from the group of the element in the Periodic Table; transition metal elements can form more than one ion.
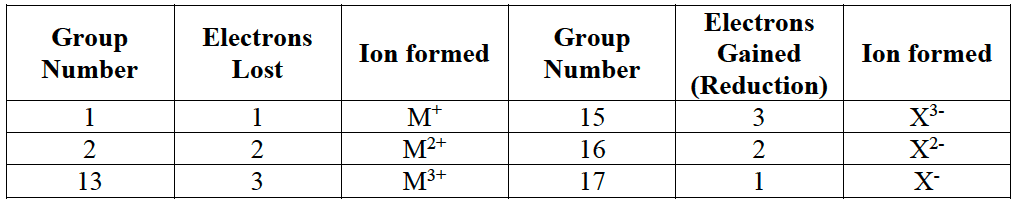
- Common polyatomic ions include: $\rm OH^-$, $\rm HCO_3^–$, $\rm NO_3^-$, $\rm CO_3^{2-}$, $\rm SO_4^{2-}$, $\rm PO_4^{3-}$.
- $\rm NH_4^+$ is unusual in that it is a positive ion formed from non-metal elements.
Ionic Compounds
- Ionic compounds consist of ions held together by forces of electrostatic attraction and are electrically neutral, as they consist of a lattice in which the total number of positive charges is balanced by the total number of negative charges.
- The formula of the compound is expressed as its simplest ratio, $\rm e.g.$ the ions $\rm X^{m+}$ and $\rm Yn^-$ will form the compound $\rm XnYm$. The formula if an ionic compound is also the empirical formula.
- In the ionic lattice, each ion is surrounded by a fixed number of ions of the opposite charge, known as the coordination number.
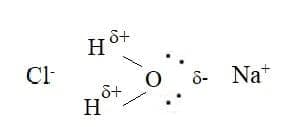
- Ionic compounds usually have high melting and boiling points, and are more soluble in polar solvents such as water than in nonpolar solvents. Positive ions ate attracted to the $\rm O$ atoms in a water molecule which have a partial negative charge, negative ions are attracted to the $\rm H$ atoms, which have a partial positive charge:
- Ions are not free to move in the solid state, so ionic compounds do not conduct electricity in the solid state.
- Ionic compounds conduct electricity when molten or in aqueous solution as the ions are free to move. They do not conduct in the solid state as the ions are fixed in a lattice structure.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
