Physical and chemical properties depend on the ways in which different atoms combine.
Elements Mixtures and Compounds
- Elements are single substances, composed of atoms of the same type.
- Compounds contain a fixed ratio of atoms of different elements and have different properties from their component elements.
- Mixtures contain more than one element or compound that are not chemically combined.
- Substances can be mixed in any proportion. Mixtures can be homogeneous with uniform properties throughout or heterogeneous in which the composition varies and components may be in different phases. A gas mixture is homogenous, sand and water form a heterogenous mixture.
- Mixtures can usually be separated by physical processes such as filtration or distillation. However, when substances react to give a chemical compound, their proportions are fixed in a stoichiometric ratio and they can only be separated again by a reaction.
Kinetic-molecular theory
- Kinetic-molecular theory describes the differences in the properties of solids, liquids, and gases on the basis of the movement and arrangement of the particles.
- Temperature in kelvin is a measure of average kinetic energy.
- State symbols indicate the state of a substance: (s) solid, (l) liquid, (g) gas and (aq) aqueous solution (dissolved in water).
 |
 |
 |
|
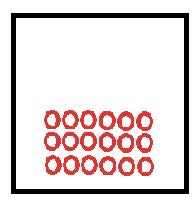
Particles vibrate about fixed position as held together together by attractive forces. |
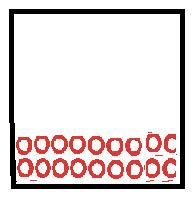
Particles move in fixed volume as particles held closely together by attractive forces. |
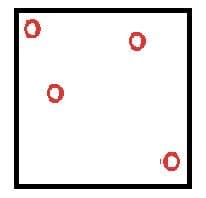
Particles can move freely as negligible attractive forces. |
- Every substance changes state by melting/freezing and boiling/condensing at a defined temperature at constant pressure. During a phase change the temperature change does not change although the substance may continue to be heated. The heat is used to change the separation between the particles rather than their kinetic energy.
- More heat is needed to convert a liquid into a gas than a solid into a liquid as more separation occurs in boiling than melting.
The temperatures that occur when a solid is heated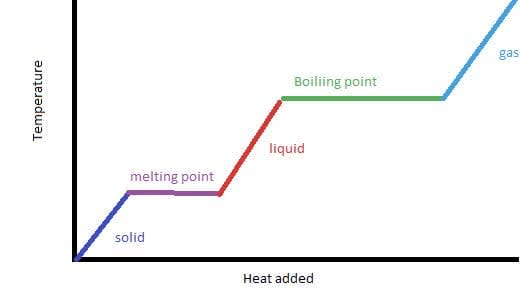
- Substances react in fixed proportions to form new substances also formed in fixed proportions. Chemical equations summarize the change when reactants are converted to products. The coefficients in a chemical equation describe the relative amounts of reactants and products.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
