The physical properties of molecular substances result from different types of forces between their molecules.
The Different types of Intermolecular Forces
- The forces between molecules are largely determined by the charge separation within the molecule:
- All molecules have London (dispersion) forces. The strength of London forces increases with the number of electrons.
- Polar molecules have additional dipole–dipole attraction.
- Van der Waals forces refer to London (dispersion) and dipole–dipole attractions.
- Polar molecules in which $\rm H$ is bonded to $\rm O$, $\rm N$, or $\rm F$ have hydrogen bonding.
- The order of relative strength:
- London (dispersion) < dipole–dipole < hydrogen bonding
- The stronger the intermolecular force, the lower the volatility (higher boiling point).
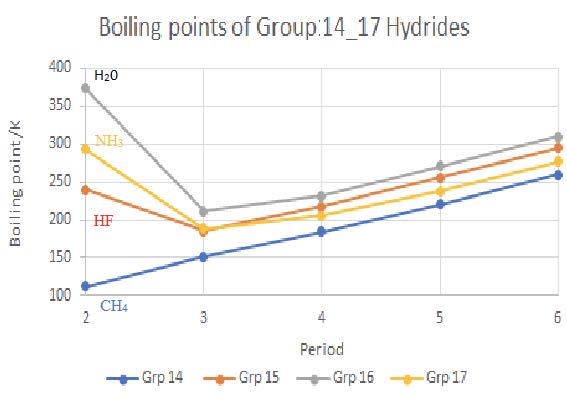
Evidence for the relative strength of hydrogen bonding comes from the relative boiling points of the group $\rm 14-$ group $17$ hydrides which highlight the relative high boiling points of $\rm H_2O$, $\rm NH_3$ and $\rm HF$.
Physical Properties
The anonymous physical properties of water and the low density of ice can be explained by the presence of hydrogen bonding.
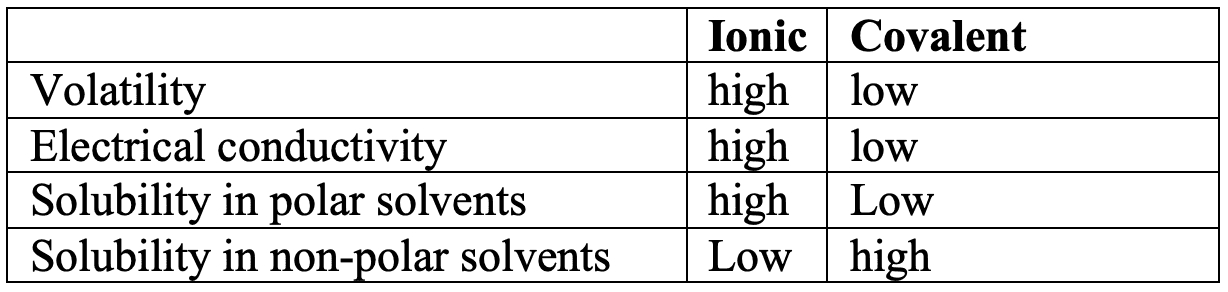
- Polar substances are more soluble in water and less soluble in non-polar solvents.
- Covalent compounds are generally not good electrical conductors, unless they are able to ionize in solution $\rm e.g.$ $\rm HCl_{(aq)}$.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
