Hybridization results from the mixing of atomic orbitals to form the same number of new equivalent hybrid orbitals that can have the same mean energy as the contributing atomic orbitals.
Methane Ethene and Ethyne
- Carbon has the ground state electron configuration $\rm 1s^22s^2sp^2$. This configuration has only $2$ unpaired electrons. To form $4 \sigma$ bonds in methane, $\rm CH_4$, an electron from the $\rm 2s$ orbital is excited to the empty $\rm 2p$ orbital. The $4$ orbitals then mix so that an electron is available for $\sigma$ bonding in four identical hybrid orbitals.
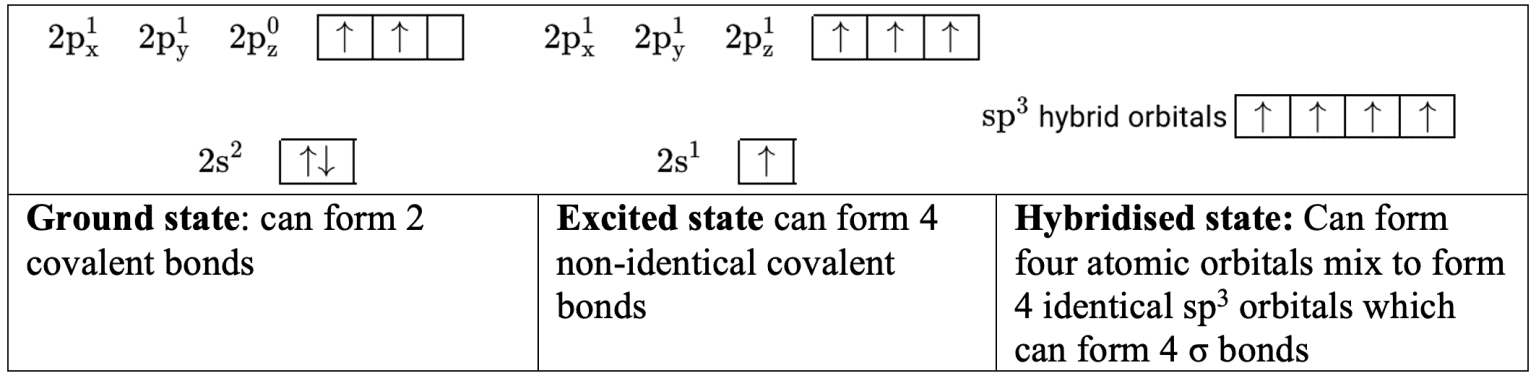
- To form $3\sigma$ bonds in ethane, $\rm C_2H_4$; the $\rm 2$s and two $\rm 2p$ orbitals mix so that an electron is available for σ bonding in three identical $\rm sp^2$ hybrid orbitals. This leaves an electron in a $\rm 2p$ orbital which is available to form a $\pi$ bond.
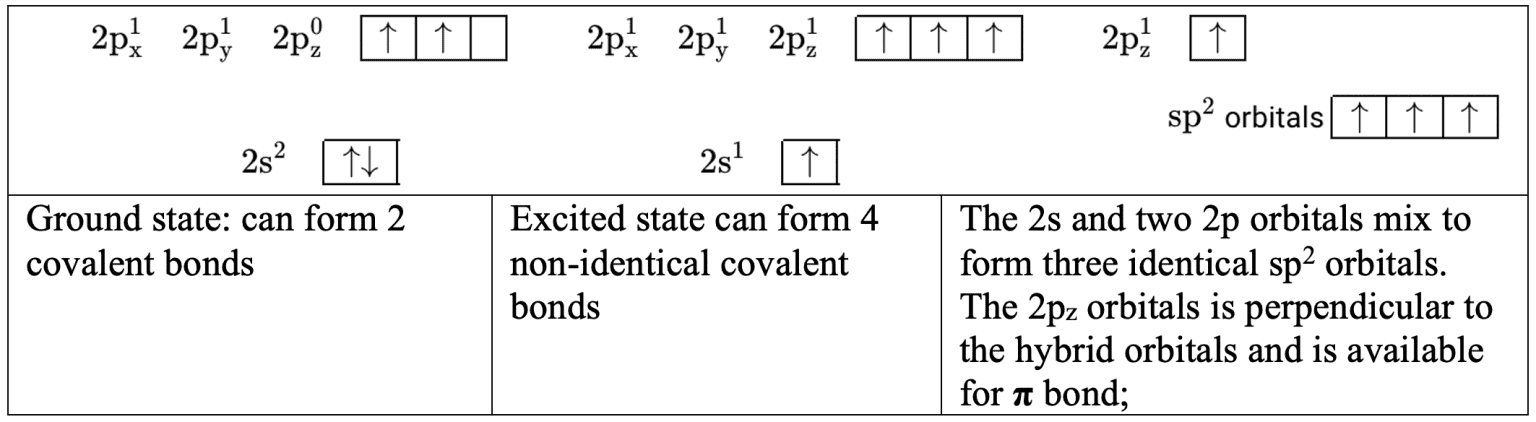
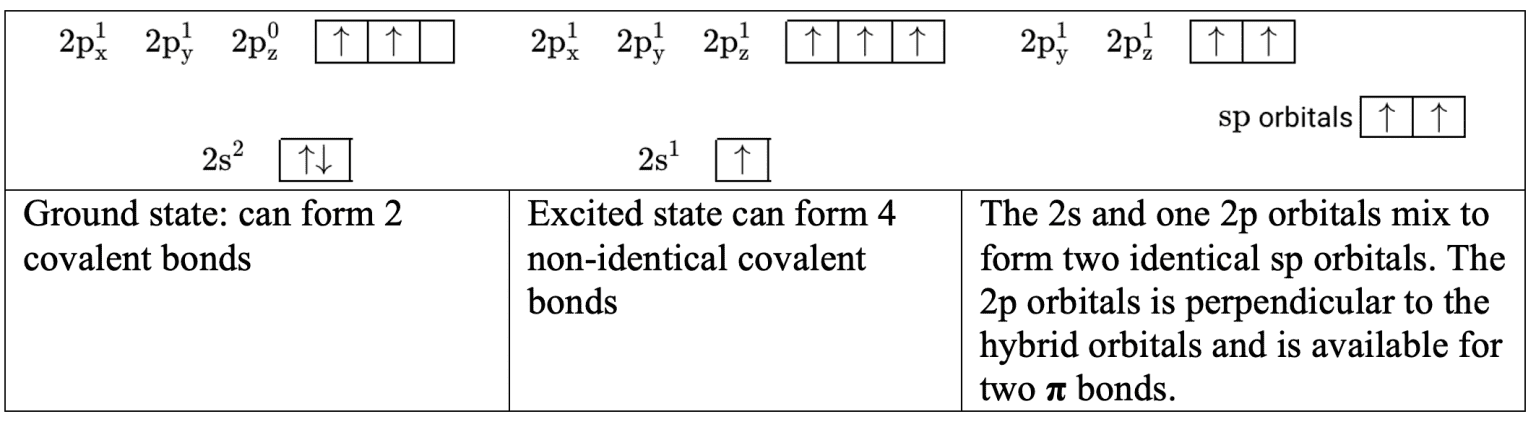
- To form $2\sigma$ bonds in ethyne, $\rm C_2H_2$, the $\rm 2s$ and one $\rm 2p$ orbitals mix so that an electron is available for $\sigma$ bonding in two identical sp hybrid orbitals. This leaves two electrons in two $\rm 2p$ orbitals which are available to form two $\pi$ bonds.
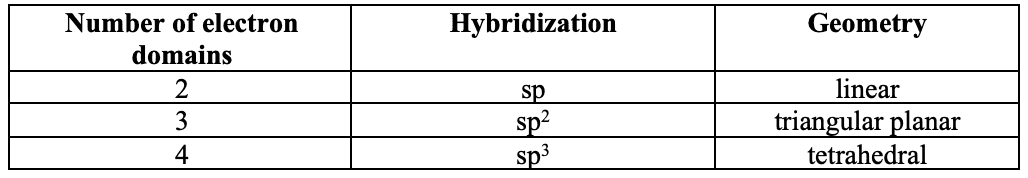
Hybridisation and geometry
- Hybridization occurs when different atomic orbitals from the same atom mix to form new atomic orbitals for bonding.
- The type of hybridization is deduced from the number of electron domains.
- Non-bonding pairs can occupy hybrid orbitals. $\rm E.g$, The two oxygen non-bonding pairs in $\rm H_2O$ occupy $\rm sp^3$ hybrid orbitals.
Worked Example
Deduce the type of hybridization shown by the carbon and oxygen atoms in ethanoic acid.
Solution
First draw the Lewis structure:
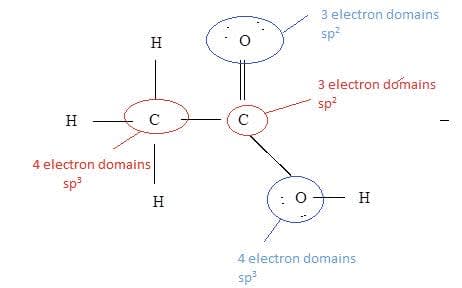
The hybridization can then be deduced by the number of electron domains.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
