In chemical transformations energy can neither be created nor destroyed (the first law of thermodynamics).
- Hess’s law states that the total enthalpy change for a reaction is independent of the route taken. It is a special case of the law of conservation of energy.
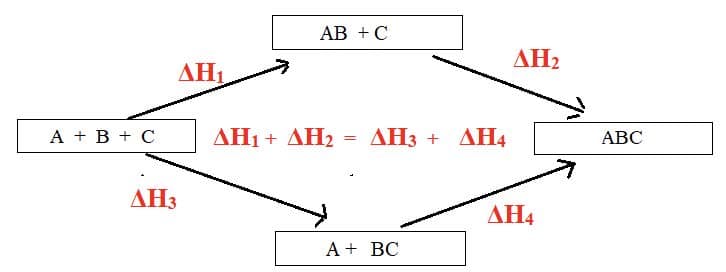
eg: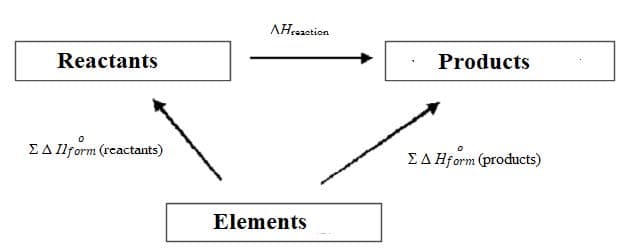
$\scriptstyle\rm \sum \Delta H^o_{form} (reactants)~ +~ \Delta H_{reaction} ~= ~\rm\sum \Delta H^o_{form} (products)$
Worked Example
Determine the enthalpy change for reactions.
$\rm PbO_2(s) + C(s) \rightarrow CO_2(g) + Pb(s)$
From the data:
![]()
Solution
Method 1
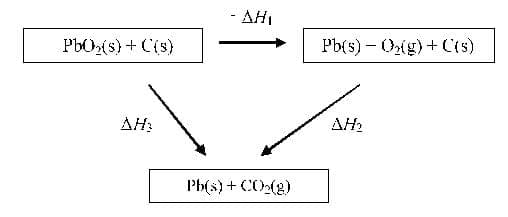
From the energy cycle:
$\rm\Delta H_3 = -\Delta H_1 + \Delta H_2$
$= +248 + -394$
$\rm\Delta H_3 = -146~kJ~mol^{-1}$
Method 2
Write the equation with the enthalpy of formation data.
$\rm \underset{-248}{PbO_2(s)} + \underset{0}{C(s)} \rightarrow \underset{-394}{CO_2(g)} + \underset{0}{Pb(s)}$
$\scriptstyle \rm\Delta H_{reaction} = \sum \Delta H^o_{form} (products)~-~ \sum\Delta H^o_{form} \overset{\Delta H_{formation}}{(reactants)}$
$\displaystyle \rm\Delta H_{reaction} = -394 - -248$ $ = \bf-146~kJ~mol^{-1}$
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
