Larger structures and more in-depth explanations of binding systems often require more sophisticated concepts and theories of bonding.
Sigma and Pi bonds
- Sigma $(\sigma)$ bonds form when atomic orbitals ($\rm s$, $\rm p$, or hybridized) overlap along the bond axis; all single bonds are $\sigma$ bonds.
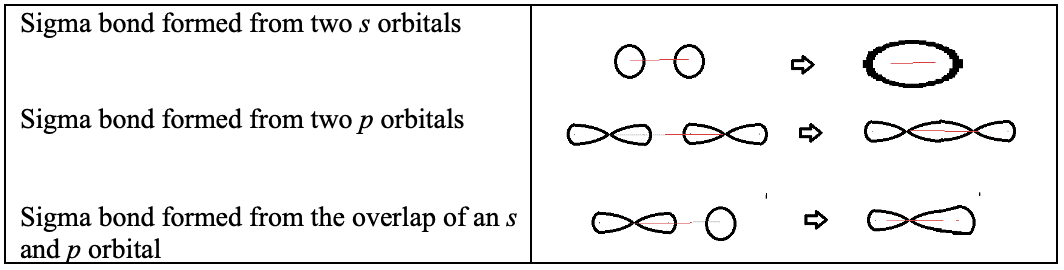
- Pi $(\pi)$ bonds form when $p$ atomic orbitals overlap laterally; the electron density is concentrated above and below the bond axis.

- Double bond = one $\sigma$ bond and one $\pi$ bond.
- Triple bond = one $\sigma$ bond and two $\pi$ bonds.
Expanding the octet
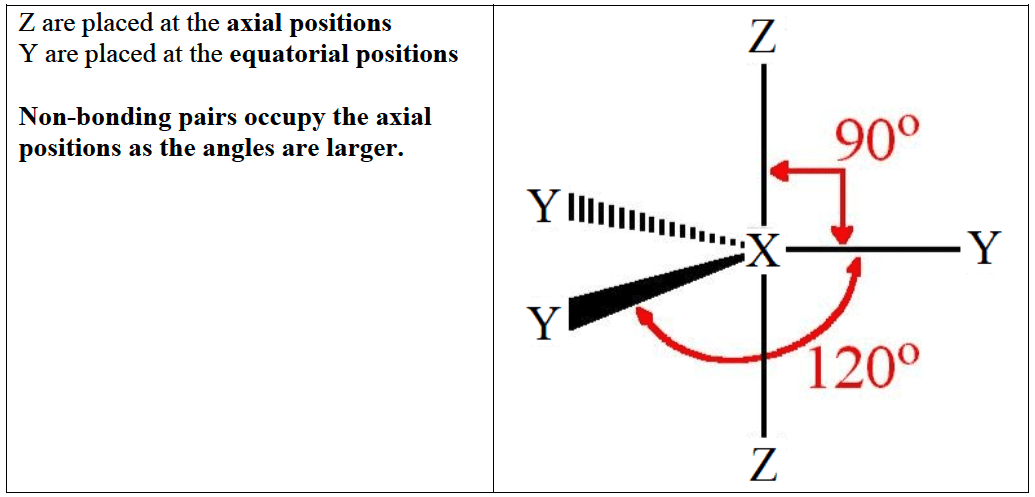
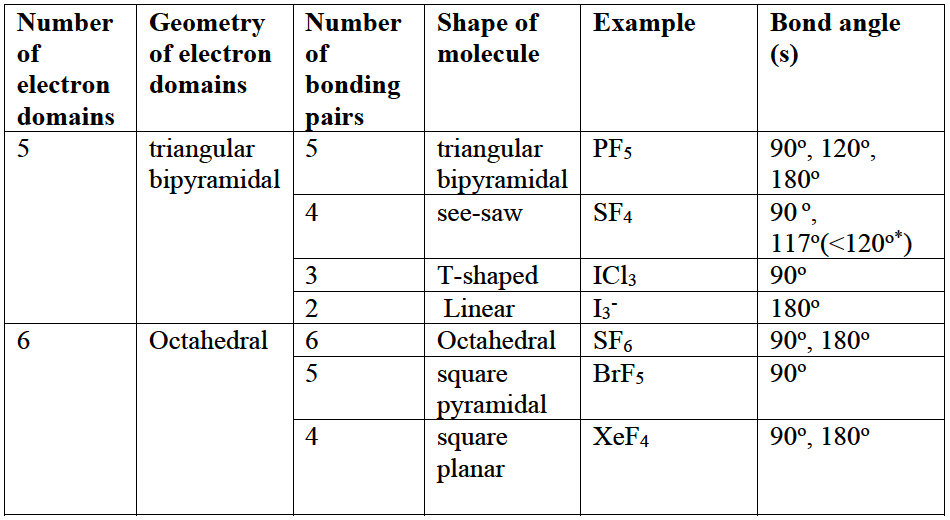
- Atoms in Period $3$ and below can expand their octet using unoccupied d orbitals. This gives rise to molecules with $5$ or $6$ electron domains around the central atom.
- In geometries involving five electrons domains the lone pairs (represented by $\rm E$) in the following systems, $\rm XY_4(nbp)$ ($4$ bonding pairs and $1$ non-bonding pair $\rm (nbp)$), $\rm XY_3(nbp)_2$ ($3$ bonding pairs and $2$ non-bonding pair $\rm (nbp)$) and $\rm XY_2nbp_3$ ($\rm 2~BPs$ and $3$ non-bonding pairs), always occupy the equatorial positions and not the axial positions.
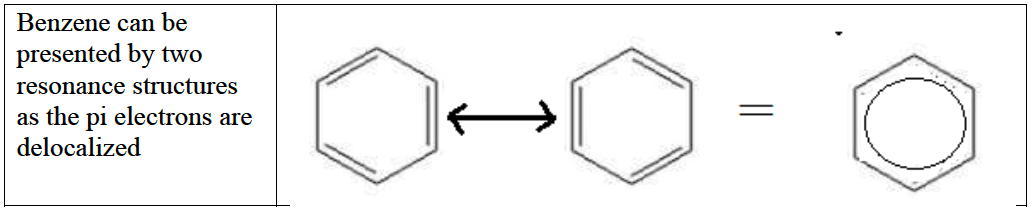
Delocalisation and Resonance
- Delocalization of $\pi$ electrons leads to greater stability and bonds of intermediate length and strength.
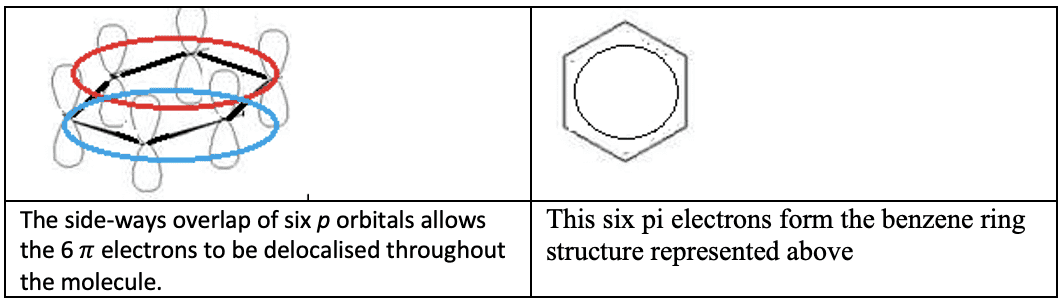
- The number of resonance structures that can be drawn for a molecule is the same as the number of possible positions for a double bond.
Formal charge
- The formation of coordinate bonds leads to non-zero formal charges. The atom which donates its non-bonding electrons to the bond has a positive formal charge and the atom which has accepted the lone pair as a bond has a negative formal charge.
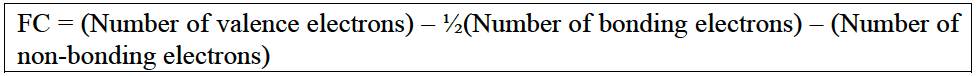
In $\rm C \equiv O$, for example, the oxygen has donated one its non bonging pairs to from a bond. It has effectively lost half a share of two electrons and so has a positive formal charge, whereas the $\rm C$ has gained half a share of the two electrons in the bond and so has a formal charge of $-1$.
$\rm FC(C) = 4 - ½ (6) - 2 = 4 - 5 = -1$
$\rm FC (O) = 6 - ½ (6) - 2 = 6 - 5 = +1$ - Formal charge $\bf (FC)$ can be used to determine which of some possible structures is the preferred structure. The most stable structure is the one with the lowest values for formal charge for the atoms.
Ozone has a bond order of $\bf 1.5$
- Ozone is a resonance hybrid with a bond order of $1.5$. Oxygen is a diatomic molecule with a bond order of $2$.
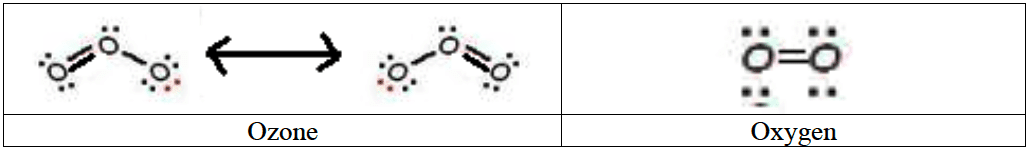
- Ozone is dissociated by light of longer wavelength/lower energy than oxygen as the bond in ozone is weaker than in oxygen.
- The catalytic breakdown of ozone by $\rm CFCs$ and $\rm NO_x$ has contributed to significant depletion of the ozone layer.
- $\rm Cl\cdot$ and $\rm NO\cdot$ are free radicals which react with ozone molecules in the stratosphere. They are regenerated during the process to break down more molecules. The overall reaction is: $\rm 2O_3 \rightarrow 3O_2$.
- $\rm Cl\cdot$ are produced by the homolytic fission of the $\rm C~ –~ Cl$ bond in $\rm CFCs$.
$\rm CCl_2F_2 \rightarrow CClF_2\cdot + Cl\cdot$
$\rm Cl\cdot + O_3 \rightarrow ClO\cdot + O_2$.
$\rm ClO\cdot + O_3 \rightarrow 2O_2 + Cl\cdot$.
$\rm NO/NO_x/NO_2$ as catalysts.
$\rm NO\cdot + O_3 \rightarrow NO_2\cdot + O_2$.
$\rm NO_2\cdot + O \rightarrow NO\cdot + O_2$.
Worked example
A Lewis structure for one of the resonance structures of ozone is shown.
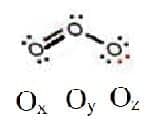
Deduce the formal charge on the three $\rm O$ atoms: in $\rm O_x$, $\rm O_y$ and $\rm O_z$.
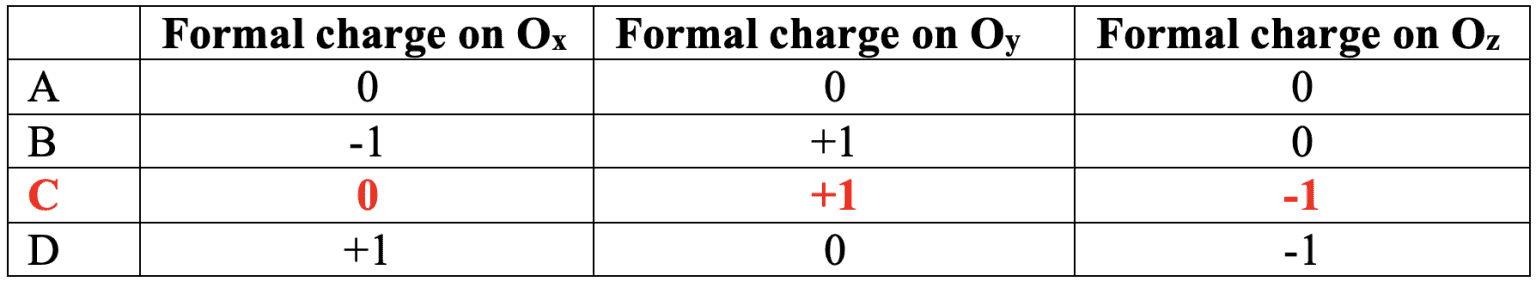
Solution
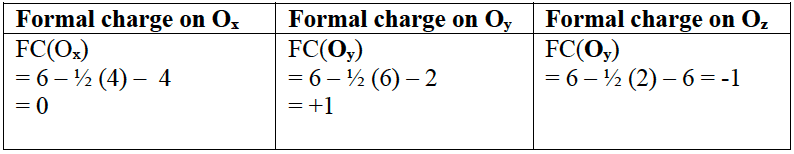
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
