Organic chemistry focuses on the chemistry of compounds containing carbon.
- A homologous series is a series of compounds with the same general formula, with a difference of $\rm –CH_2–$ between successive members.
- Members of the same homologous series show a trend in their physical properties and have similar chemical properties.
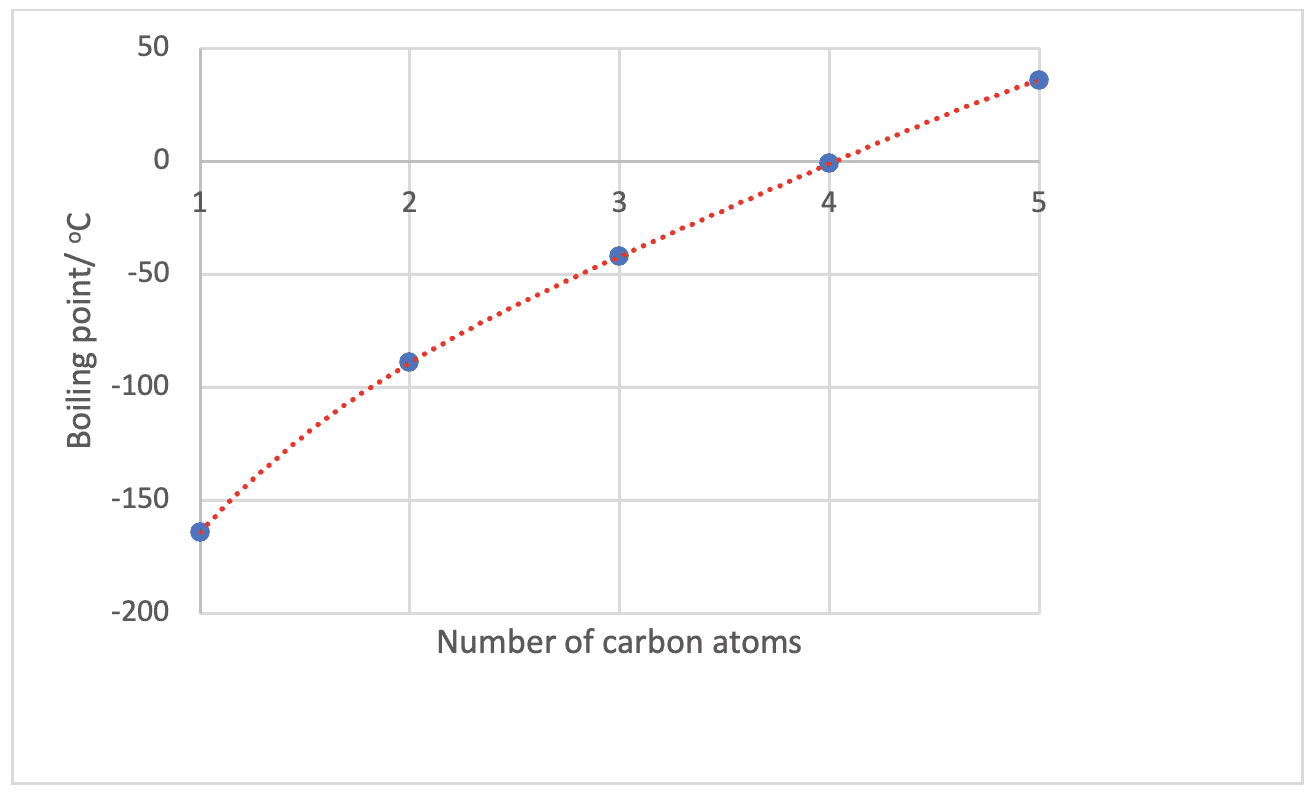
The boiling points of the alkanes increase as the number of carbon atoms increase.
- Different formulas are used to describe organic compounds:
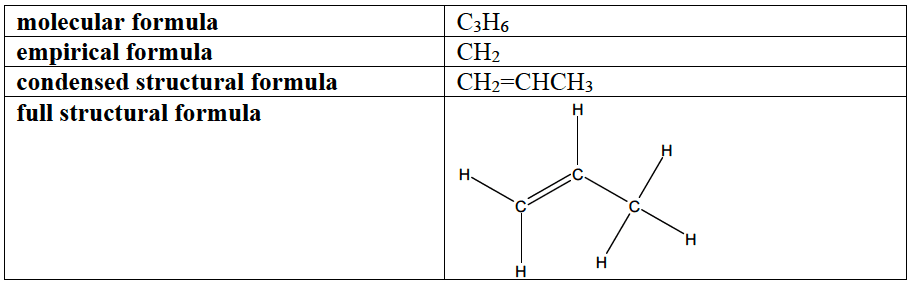
- molecular formula is the actual number of atoms present in a molecule
- empirical formula is the simplest ratio of atoms present in a molecule
- condensed structural formula shows all the atoms and their positions without showing single covalent bonds. All monovalent substituents are grouped together. Double and triple bonds may be shown depending on the context.
- full structural formula shows all the bonds in a molecule
- stereochemical formula shows the 3-dimensional arrangement of the atoms.
(Note: Skeletal formula which shows all covalent bonds as lines but omit carbon and hydrogen atoms, is not a structural formula as it does not include all the hydrogen atoms.)
Different formula for Propene
Full structural and stereochemical formula $\bf CH_3Cl$

- Saturated compounds contain single bonds only, and unsaturated compounds contain at least one double or triple bond.
IUPAC nomenclature is used to describe organic compounds.
- Stem: named for the longest carbon chain where:
- $\rm C_1~meth-$, $\rm C-2~eth-$, $\rm C_3~prop-$, $\rm C_4~but-$, $\rm C_5~pent-$, $\rm C_6~hex-$, $\rm C_7~hept-$, $\rm C_8~oct-$
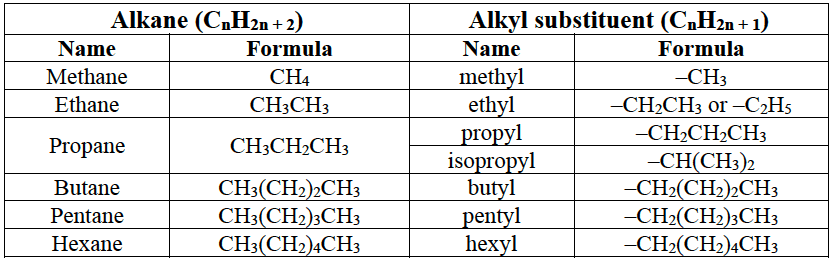
Alkanes and alkyl substituents.
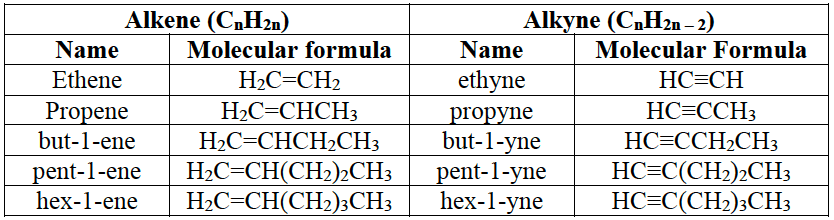
Alkenes and alkynes.
- Suffix used for the functional group ending:
-ene, -anol, -anal, -anone, -anoic acid, -anoate, -anamide, -anamine, -anenitrile, -benzene - Prefix used for substituent groups, using the smallest number to denote the main-chain C atom.
methyl-, ethyl-, propyl-, fluoro-, chloro-, bromo-, iodo-, amino- - The functional group is the reactive part of a molecule. It defines the class of compound
e.g. the hydroxyl group defines the class, the alcohols.
Common functional groups in decreasing order of priority.
Hydrocarbon substituents are denoted as R.
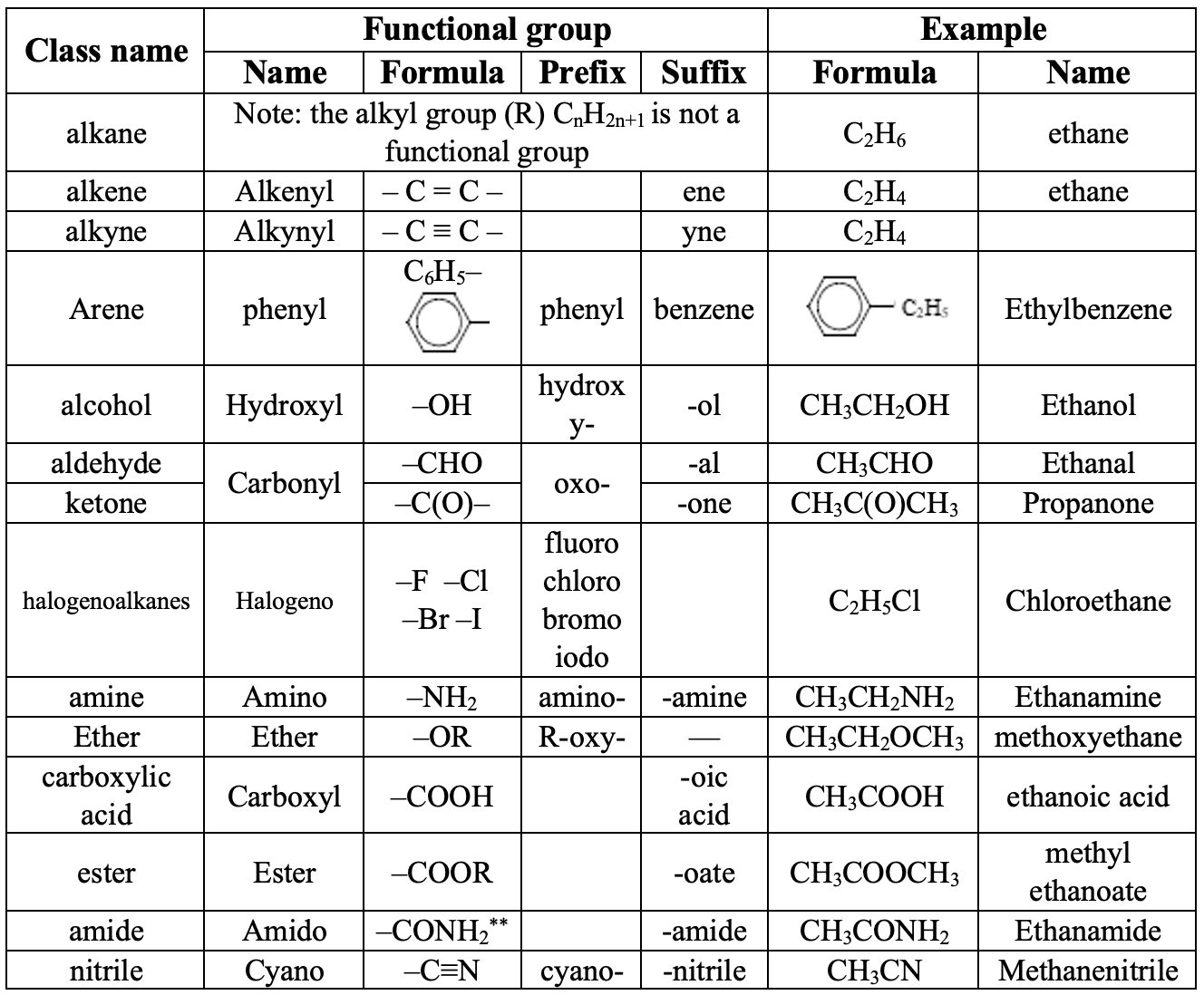
Worked example
Deduce the IUPAC name of the following compound.
Solution
This compound is a carboxylic acid, so the last suffix in the IUPAC name will be "-oic acid". The longest chain is saturated and consists of six carbon atoms, so the stem will be "hexan". The chain is numbered from the end closest to the functional group:
"3-methyl" + "hexanoic acid”
Answer = 3- methyl-hexanoic acid.
Isomers
- Structural isomers are molecules with the same molecular formula but different structural formulas. They contain atoms attached in a different order, and have distinct physical and chemical properties.
- Structural isomers can be straight/branched chains or differ in the position or nature of the functional group.
- Straight chain molecules can pack together better than branched chain molecules and generally have stronger intermolecular forces.
Worked example
Identify the order of increasing boiling point for the isomers of $\rm C_5H_{12}$.
Solution
The molecules have the same molecular formula and the same number of electrons so the dispersion forces, which depend on the number of electrons are similar.
The differences of boiling point are due to how the molecules pack together.
Molecules which are “sausage” shaped pack together more efficiently than spherically shaped molecules and so have stronger intermolecular forces.
The structural isomers have the following condensed structures:
![]()
The order of increasing boiling point is:
$\rm CH_3C(CH_3)_3$ $\rm < CH_3CH(CH_3)CH_2CH_3$ $\rm < CH_3CH_2CH_2CH_2CH_3$
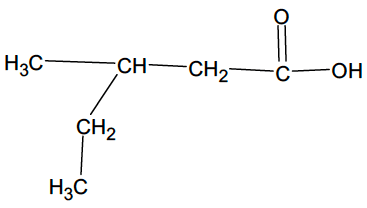
Primary, secondary and tertiary structures
- Halogenoalkanes and alcohols can be classified as primary (1°), secondary (2°) and tertiary (3°) according to the number of carbon atoms bonded to the carbon with a halogen or hydroxyl group.
- Primary, secondary, and tertiary carbon atoms are attached to a functional group, and differ in the number of hydrogen atoms to which they are also attached.
- Primary, secondary, and tertiary compounds e.g. alcohols and halogenoalkanes, show some different chemical properties.
- Amines are classified in a similar way according to the number of carbon atoms bonded directly to the nitrogen atom.
Benzene and Aromatic compounds
- Arenes contain the benzene ring. They are known as aromatic compounds. Organic compounds without the benzene ring are known as aliphatic compounds.
- Benzene $\rm (C_6H_6)$ is the simplest arene. The carbon atoms in benzene form $3$ localised bonds with their neighbouring carbon and hydrogen atoms. The $\rm 4^{th}$ electron is available for bonding in a delocalised ring that surrounds all six carbon atoms in the aromatic ring. This arrangement is called the benzene ring functional group and is found in many compounds.
- The bond order of each carbon–carbon bond in the benzene ring is intermediate between a single and a double bond and is $1.5$.
- The structure of benzene is commonly represented by a six‑membered ring with a circle inside:
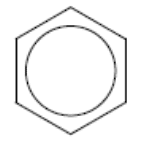
- The structure of benzene is supported by physical and chemical evidence.
Physical evidence for the structure of benzene.
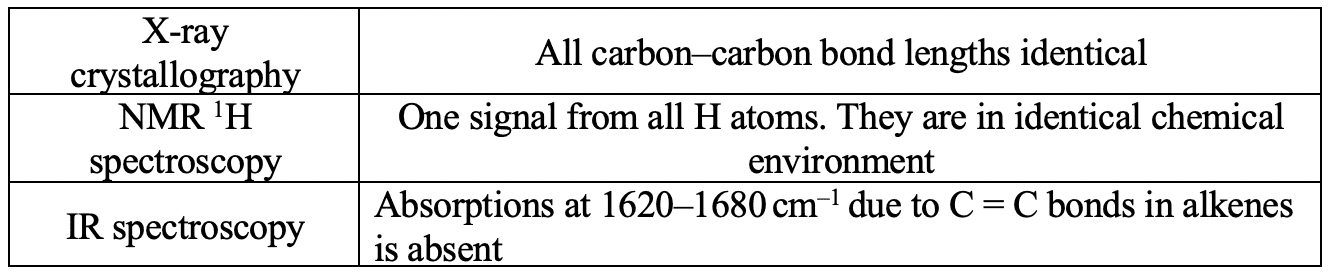
Chemical evidence for the structure of benzene.

- Benzene has distinct properties due to its delocalized $(\pi)$ electrons, which give it an extra stability. It is a planar, non-polar molecule, which does not readily undergo addition reactions despite being highly unsaturated.
The volatility of organic compounds
- The volatility of organic compounds depends on:
- The size of the molecule/length of the hydrocarbon chain and the functional group.
- The larger members of a homologous series are less volatile due to stronger London (dispersion) forces.
- More polar functional groups decrease the volatility of the compound.
- The presence of intermolecular hydrogen bonds, in particular, also decreases the volatility.
The boiling points of some alkanes, alcohols fluoroalkanes and iodalkanes
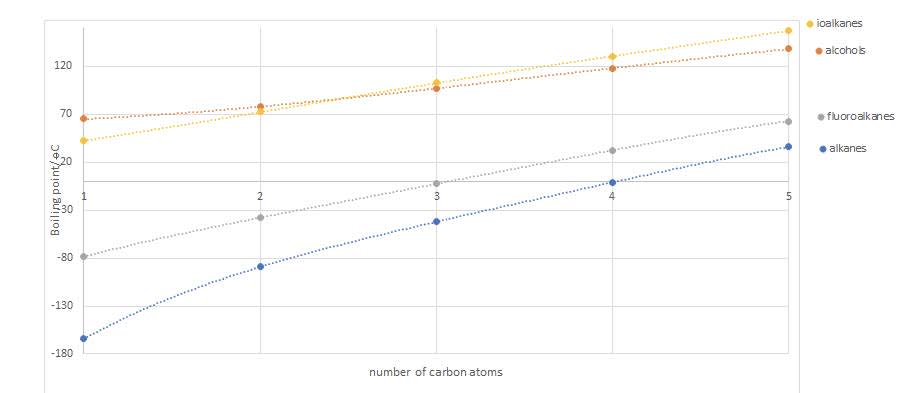
- Some points to note:
- A flouroalkane has a higher boiling point than the corresponding alkane due to dipole-dipole interactions.
- An alcohol has a higher boiling point than the corresponding alkane as there is intermolecular hydrogen bonding.
- An iodoalkane has a higher boiling point than the corresponding flouroalkane as they have stronger dispersion forces due to the larger number of electrons on the iodine than fluorine, which more than compensates for the weaker dipole-dipole interactions.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
