Structure, bonding and chemical reactions involving functional group interconversions are key strands in organic chemistry.
Hydrocarbons contain carbon and hydrogen only.
- Hydrocarbons contain carbon and hydrogen only. Alkanes, alkenes, alkynes, and benzene are hydrocarbons.
- The combustion of hydrocarbons is an exothermic reaction, but these reactions have high activation energy, which explains the (kinetic) stability of the compounds and their existence as valuable fuels.
- In excess $\rm O_2$ complete combustion occurs and $\rm CO_2$ and $\rm H_2O$ are produced.
- In limited $\rm O_2$, incomplete combustion occurs and $\rm CO$ or $\rm C$, and $\rm H_2O$ are produced.
- The burning of hydrocarbons has harmful effects on the environment and health.
Alkanes are saturated hydrocarbons.
- Alkanes are saturated hydrocarbons.
- They have low reactivity as the $\rm C–C$ and $\rm C–H$ bonds are strong and non-polar.
- Alkanes undergo free radical substitution reactions with halogens in uv light. Radicals are reactive species (atom, molecule or ion) with an unpaired valence electron.
- The halogen undergoes photochemical homolytic fission to produce free radicals in the initiation step.
$\rm X_2 \overset{\mathcal h}{\longrightarrow} 2X~\bullet$ - The radicals substitute for $\rm H$ in the alkanes in propagation reactions that also produce free radicals. The reaction produces a mixture of substitution products.
$\rm R- H + X~\bullet \rightarrow R\bullet + HX$
$\rm R\bullet + X_2 \rightarrow RX + X~\bullet$ - Termination steps involve two free radicals joining together.
$\rm R\bullet\quad +\quad X~\bullet \quad \rightarrow \quad R – X$
$\rm R\bullet \quad + \quad R~\bullet\quad \rightarrow \quad R – R$
$\rm X\bullet \quad + \quad X~\bullet \quad \rightarrow \quad X_2$
- The halogen undergoes photochemical homolytic fission to produce free radicals in the initiation step.
Alkenes are unsaturated hydrocarbons
- Alkenes are unsaturated hydrocarbons containing a carbon–carbon double bond. Alkenes are more reactive than alkanes.
- The double bond is the site of reactivity as one of the bonds $\rm (AHL: \pi)$ breaks relatively easily, and so alkenes are more reactive than alkanes.
- Alkenes undergo addition reactions, by breaking their double bond.
- Addition reactions of alkenes include:
- addition of $\rm H_2$, hydrogenation, to produce alkanes. Hydrogenation of alkenes is catalysed by transition metals (such as $\rm Ni$ or $\rm Pt$) and requires high temperature and pressure.
- addition of halogens to produce dihalogenoalkanes
- addition of hydrogen halide, hydrohalogenation, to produce halogenoalkanes
- addition of $\rm H_2O$, hydration, to produce alcohols. Hydration proceeds in the presence of concentrated inorganic acids, such as $\rm H_3PO_4$ or $\rm H_2SO_4$.
- Alkenes decolorize bromine water in the dark or light, and this colour change can be used to distinguish between alkanes and alkenes.
- Alkenes undergo addition reactions to form addition polymers by breaking their double bonds. The repeat unit shows the structure of the monomer with open bonds on each side.
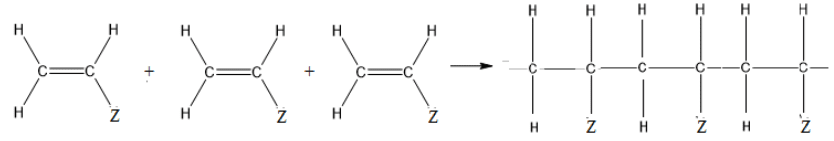
Addition polymerisation is catalysed by free radicals or transition metal compounds at high temperatures.
Alcohols
- The complete oxidation of the alcohol molecules in combustion produces $\rm CO_2(g)$, $\rm CO(g)$, $\rm C(s)$ due to breaking of $\rm C – C$ bonds. Selective oxidation using oxidising agents only changes the functional group.
- Alcohols are fuels and, like hydrocarbons, yield products ($\rm CO_2(g)$, $\rm CO(g)$, $\rm C(s)$ that depend on the amount of oxygen available.
- Oxidizing agents include acidified potassium dichromate(VI) or potassium manganate(VII) with a heated reaction mixture.
- Alcohols differ in their ability to be oxidized. The degree of oxidation can be related to the number of H atoms attached to carbon with the hydroxyl group.
- Primary alcohols are first oxidized to an aldehyde, and with prolonged oxidation to a carboxylic acid. The aldehyde product can be separated by distillation as it has the lowest boiling point in the mixture. For prolonged oxidation to carboxylic acid, reflux is used. The oxidizing agent changes colour as it is reduced.
- Secondary alcohols are oxidized to a ketone. The oxidizing agent changes colour as it is reduced.
- Tertiary alcohols cannot be selectively oxidised by potassium dichromate(VI) or potassium manganate(VII). The oxidizing agent does not change colour. Any oxidation involves breaking the carbon chain as in combustion.
- Alcohols react with carboxylic acids to produce an ester and water (esterification). Concentrated sulfuric acid is used as a catalyst in the reaction.
Esterification: $\rm acid + alcohol \overset{H'}{\rightleftharpoons} ester + water$
$\rm RCOOH + R⸝OH \overset{H'}{\rightleftharpoons} RCOOR⸝ + H_2O$ - Esterification is a condensation reaction. Condensation reactions involve two or more reactants that combine together to produce a larger molecule and release a small molecule, such as water, as a by-product.
Halogenoalkanes RX contain the polar $\bf C–X$ (C–halogen) bond
- Halogenoalkanes are more reactive than alkanes due to the polar $\rm C– X$ bond.
- Halogenoalkanes react with nucleophiles which are attracted to the electron-deficient carbon of the $\rm C–X$ bond.
- Nucleophiles that attack the halogenoalkanes are species that possess a lone pair of electrons. They are Lewis bases and can also possess a negative charge.
- Nucleophiles with a negative charge are more reactive than the corresponding uncharged molecule. ($\rm e.g. :OH^-$ is more reactive than: $\rm OH_2$)
- Halogenoalkanes undergo substitution reactions where the halogen is replaced by a nucleophile. ($\rm e.g.$ Halogenoalkanes react with $\rm NaOH(aq)$ to form an alcohol).
$\rm e.g. RCl + NaOH \rightarrow ROH + NaCl$
Benzene undergoes substitution reactions
- Benzene does not readily undergo addition reactions, but instead undergoes electrophilic substitution reactions that preserve the stable ring structure.
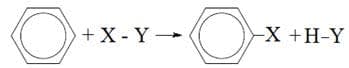
where $\rm X – Y$
$\rm = Cl – Cl$ with $\rm Fe$ or $\rm AlCl_3$ as a catalyst
$\rm = HO - NO_2 (HNO_3)$ with concentrated sulfuric acid as a catalyst. - Electrophiles are electron-deficient species that are attracted to the electron-dense benzene ring.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
