The quantized nature of energy transitions is related to the energy states of electrons in atoms and molecules.
Electron configuration and first ionisation energies of the elements
- The electron configuration of an atom describes the number of electrons in each energy sub-level.
- Line emission spectra provide experimental evidence for the existence of atomic energy levels. The lines converge at higher energies, forming a continuum. The frequency of the radiation at the limit of convergence corresponds to the first ionization energy, $\rm IE_1$.
- The first ionization energy of an element is the minimum energy required to remove one mole of electrons from a mole of gaseous atoms to form a mole of univalent cations in the gaseous state. It is the enthalpy change for the reaction: $\rm X(g) \rightarrow X^+(g) + e^-$
- Evidence for the existence of main energy levels and sub-levels comes from graphs of first ionization energies of successive elements and successive ionization energies of the same element.
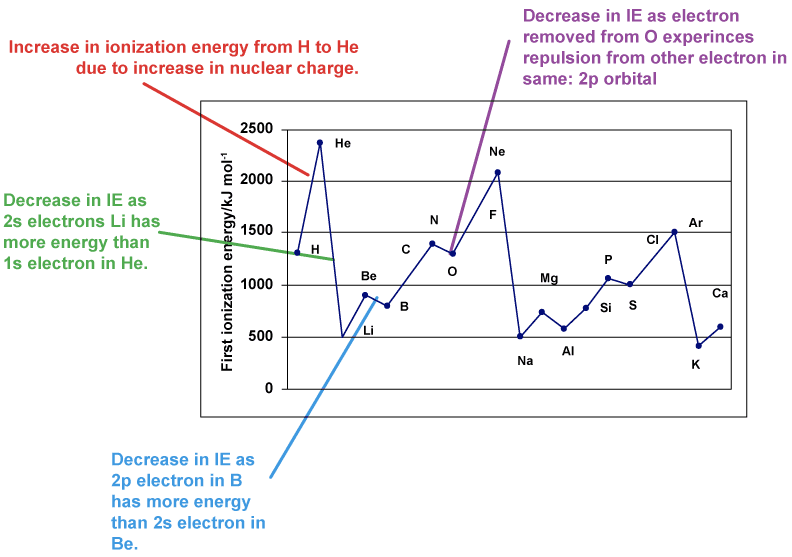
- First ionizations decrease down a group due to increasing distance of outer electrons from the nucleus which reduces the electrostatic attraction between the nucleus and the outer electrons.
- There is a general increase in $\rm IE_1$ across a period. The increase in nuclear charge results in a stronger attraction between the nucleus and the outer electrons. Decreases occur, however, when the electron is removed from a higher sublevel or when the electron is removed from an orbital which is doubly occupied (note the drop between $\rm p^3$ to $\rm p^4$. Paired electrons are easier to remove than unpaired electrons due to the repulsion between the electrons in the same orbital. The repulsion between the electrons in the same orbitals makes the electrons easier to remove than unpaired electrons.
Electron configuration and successive ionisation energies of the elements
- Successive ionization energies relate to removing electrons from a gaseous species. $\rm IE_2$ is the enthalpy change for the process: $\rm X^+(g) \rightarrow X^{2+}(g) + e^{-}$.
- There is an increase in successive ionization on energies as the electrons are increasing more difficult to remove from a positive ion with increasing charge.
- More significant increases in successive ionisation occur when an electron is removed from a different energy level or different sub-level. A very small jump occurs when there is a change from a $\rm p^4$ to $\rm p^3$ configuration.
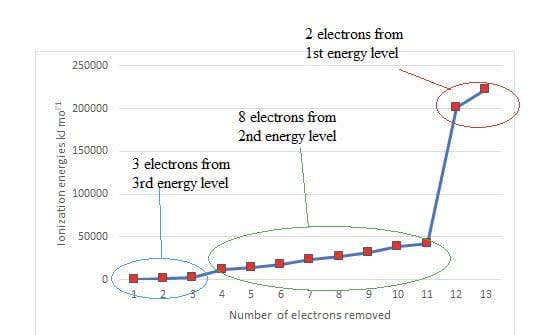
The ionization energies of Aluminium reflects the occupancy of the first three energy levels.
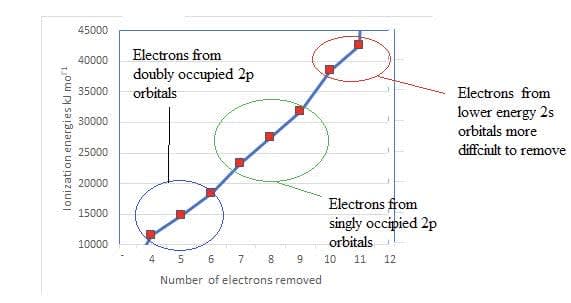
A closer look shows the occupancy of the electrons in sublevels in the second energy level.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
