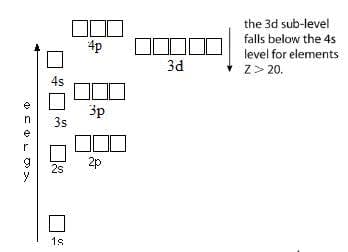
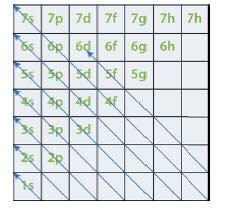
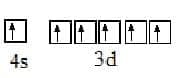
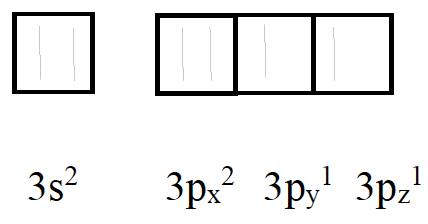The electron configuration of an atom can be deduced from its atomic number
- The electromagnetic spectrum includes in order of increasing frequency/energy:
radio waves, microwaves, IR radiation, visible light, ultraviolet radiation $\rm X-$rays, and $\gamma$ rays. (See section $3$ of the IB data booklet) - The frequency $(v)$ and wavelength $[\lambda]$ are related by: $\rm c$ (speed of light] $= \lambda v$.
- The energy of a photon of light $\rm E_{photon}$ is related to the frequency $[v]$ of the radiation by Planck's equation:
$\mathrm{E_{photon}} = h\nu = \dfrac{hc}{\gamma}$, (the equation is given in section $1$ of the IB data booklet)
$\rm h$ is Planck's constant $\rm 6.63 \times 10^{-34}~Js.h$ is Planck’s and $\rm c$ is the speed of light $\rm = 3.00 \times 10^8~m~s^{-1}$ (see section $2$ of the IB data booklet}. - A continuous spectrum contains radiation of all wavelengths within a given range ($\rm e.g.$ the visible spectrum).
- A line spectrum consists of discrete lines of different wavelengths / frequencies. An emission spectrum of hydrogen atom consists of different series of lines against a dark background in different regions of the electromagnetic spectrum. An absorption spectrum is like a “negative” of a emission spectrum and consists of a series of dark lines against a coloured background.
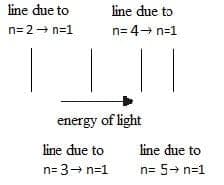
- The emission spectrum of hydrogen atom consists of different series of lines in different regions of the electromagnetic spectrum. The lines are produced when excited electrons fall from higher to lower energy levels.
Energy levels of hydrogen atom Lines in the emission spectra (Lyman series) 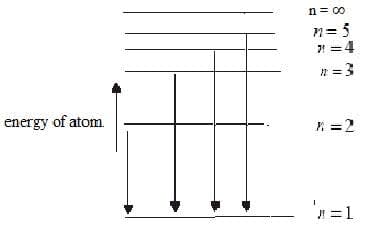
- Each photon of light is emitted when an electron falls from an excited state to a lower energy level and corresponds to a particular energy wavelength and frequency. $\mathrm{E_{photon}} = h\nu = \dfrac{hc}{\gamma}$.
- Transitions where the electron falls to the $\rm n = 1$ give out the most energy and are in the ultraviolet region. Transitions to $\rm n = 2$ are of less energy and are in the visible region and transitions to $\rm n = 3$ are in the infrared region.
- The line emission spectrum of hydrogen provides evidence for electrons occupying discrete energy levels.
- The energy levels of the hydrogen atom converge at higher energy as they are further from the nucleus, and the lines in the spectrum also converge at higher energy/frequency.
- The limit of convergence $\rm n = \infty$ corresponds the electron leaving the atom. The energy between the $\rm n = 1$ and $\rm n = \infty$ energy levels is called the ionisation energy.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA