Voltaic cells convert chemical energy to electrical energy and electrolytic cells convert electrical energy to chemical energy. Energy conversions between electrical and chemical energy lie at the core of electrochemical cells.
Voltaic cells
- Voltaic cells convert energy from spontaneous exothermic chemical processes to electrical energy. They are formed by connecting together two half-cells by a salt bridge and an external circuit.
Voltaic cell for the reaction: $\bf Zn(s) + Cu^{2+}(aq) \rightarrow Zn^{2+}(aq) + Cu(s)$
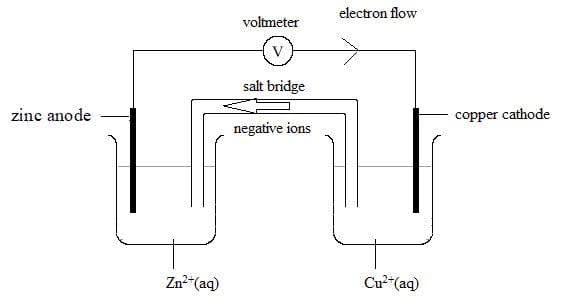
Anode half-reaction:
$\rm Zn(s) \rightarrow Zn^{2+}(aq) + 2e^-$
Cathode half-reaction:
$\rm Cu^{2+}(aq) + 2e^- \rightarrow Cu(s)$
Electrons flow from the zinc to the copper electrodes in the external circuit.
The zinc is the negative electrode, and the copper electrode is the positive electrode.
- The anode is the electrode where oxidation occurs. It has a negative charge in a voltaic cell.
- The cathode is the electrode where reduction occurs. It has a positive charge in a voltaic cell.
- Electrons flow through the external circuit of a voltaic cell from negative electrode to positive electrode: from anode to cathode.
The salt bridge completes the electrical circuit, allowing aqueous ions to move from one half cell to another. Negative ions for example will move from right to left in the above voltaic cell and positive ions will move in the opposite direction. - When writing a one-line representative cell diagram notation for a voltaic cell, the salt bridge is represented by two vertical lines, ||, and each phase boundary, by a single vertical line |. The cathode is always written on the right-hand side and the anode on the left-hand side.
E.g. For the cell above the diagram corresponding to the reaction:
$\rm Zn(s) + Cu^{2+}(aq)$ $\rm \rightarrow Zn^{2+}(aq) + Cu(s)$ is $\rm Zn(s)|Zn^{2+}(aq) \| Cu^{2+}(aq)|Cu(s)$
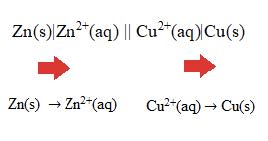
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
