Energy conversions between electrical and chemical energy lie at the core of electrochemical cells.
Voltaic cells
- If two half-cells are connected to a voltmeter with a salt bridge between them, the voltaic cell produces a voltage. Electrons flow through the external circuit from anode to cathode.
- The voltage measured by a voltmeter when no current is flowing is known as the cell potential, $\rm E_{cell}$.
- The higher the $\rm E^{\theta}$ value of a cell the greater the tendency for reduction to occur.
- The lower the $\rm E^{\theta}$ value of a cell the greater the tendency for oxidation to occur.
- The greater the difference in reactivity between the half-cells, the greater the $\rm E_{cell}$.
- The standard hydrogen electrode is used as the reference standard for voltaic cells, and is assigned a value of $\rm 0.00~V$.
- The standard electrode potential of a half-cell is measured with reference to the standard hydrogen electrode, operating under standard conditions($\rm 1~mol~dm^{-3}$ for reactants $(\rm e.g. H^+(aq))$ in solution and $\rm 100~kPa$ for gaseous reactants $\rm (e.g.$ $\rm H_2(g)$, $\rm Temperature = 298~ K)$.
- Using reduction potentials (with the signs as given):
$\rm E^{\theta}_{cell} = E^{\theta}_{half - \text{cell where reduction occurs}}$ $\rm − E^{\theta}_{half - \text{cell where oxidation occurs}}$ - The cell potential, $\rm E^{\theta}$, is related to the change in Gibbs energy, $\rm \Delta G^{\theta}$ of the corresponding reaction:
$\rm \Delta G^{\theta}= –nFE^{\theta}$.
$n$ is the number of electrons transferred in the reaction.
$\rm F$ (Faraday’s constant) is the charge of $\rm 1~mol$ of electrons $\rm = 96~500~C~mol^{–1}$
Applying this relationship- Spontaneous reactions have negative $\rm \Delta G^{\theta}$ and positive $\rm E^{\theta}$.
- Non-spontaneous reactions have positive $\rm \Delta G^{\theta}$ and negative $\rm E^{\theta}$.
- Reactions at equilibrium have $\rm \Delta G^{\theta}$ and $\rm E^{\theta} = 0$.
Electrolytic cells
Products at Anode and Cathode
- Electrolysis occurs when ions can move, so the electrolyte (liquid with ions) must be in the molten or in aqueous solution. This section focusses on the aqueous solution.
When a molten ionic compound is electrolysed the constituent ions are oxidised and reduced. When an aqueous compound is electrolysed the water present may also be oxidised or reduced. - In aqueous solution water can be oxidized to oxygen at the anode:
$\rm H_2O(l) \rightarrow ½ O_2(g) + 2H^+(aq) + 2e^−$ $\rm E^{\theta} = -1.23~V$ and reduced to hydrogen at the cathode.
$\rm H_2O(l)+ e^− \rightarrow ½H_2(g) + OH^−(aq)$ $\rm E^{\theta} = −0.83~V$. - The products of electrolysis in aqueous solution depend on:
- the $\rm E^{\theta}$ values:
At the cathode with concentrated solutions.
Hydrogen is generally produced except in the case of metals with more positive $\rm E^{\theta}$ values such as copper and silver.
At the anode with concentrated solutions.
Halogens are produced if a halide is present. Oxygen is produced from the water with other common negative ions, - the concentration of electrolyte:
The amount of hydrogen produced at the cathode and oxygen produced at the anode increase as the solutions become more dilute. - the nature of the electrode.
Generally inert electrodes are used but copper anodes are themselves oxidised in preference to any ions in solution.
- the $\rm E^{\theta}$ values:
Electrolysis of Sodium Chloride with inert electrodes at different concentrations
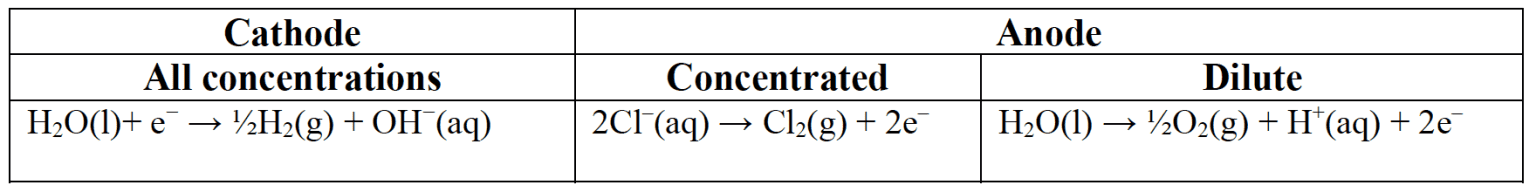
![]()
Electrolysis of copper sulfate with different electrodes
![]()
![]()
Worked Example
The electrolysis of dilute and concentrated aqueous solutions of potassium bromide result in different products. Deduce the half-reactions that occur at the anode in the different cases.
Solution
There are two species attracted to the anode: $\rm Br^-(aq)$, $\rm H_2O$ in a concentrated solution $\rm Br^-(aq)$ is oxidized to $\rm Br^2(aq)$.
This is observed experimentally. $\rm 2Br^– (aq) \rightarrow Br^2(g) + 2e^– E^{\theta} = -1.09~V$ in a dilute solution $\rm H_2O(l)$ is oxidized to $\rm O_2(g)$.
$\rm H_2O(l) \rightarrow ½O_2(g) + 2H^+(aq) + 2e^-$ $\rm E^{\theta} = -1.23~V$.
Bubbles of oxygen are observed at the anode.
(Hydrogen is produced at the cathode in both cases).
Factors affecting the amount of products
- Electrical charge delivered $\rm (C) = current~ (A) \times time~ (s)$
$\rm Q = It$ - The amount of product in electrolysis depends on:
- the ionic charge
- the current
- the time the current flows.
- The equation for the discharge of an ion shows the moles of electrons required.
$\rm e.g.$
$\rm Cu^{2+}(aq) + 2e^– \rightarrow Cu(s)$- for $\rm 1~mol$ $\rm Cu(s)$ to be produced $\rm 2~mol$ of electrons are needed.
$\rm 2Cl^–(aq) \rightarrow Cl_2(g) + 2e^–$ - for $\rm 1~mol$ $\rm Cl_2$ to be discharged $\rm 2~mol$ of electrons are needed.
$\rm 1~mol$ of electrons has a charge of $\rm 96~500~C = 1~F$.
- for $\rm 1~mol$ $\rm Cu(s)$ to be produced $\rm 2~mol$ of electrons are needed.
Electroplating
- Electrolysis can be used to electroplate objects with a thin layer of metal – the conducting object to be plated is at placed at the cathode (negative electrode), the metal used to plate the object is placed at the anode (positive electrode). The electrolyte contains ions of the metal.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
