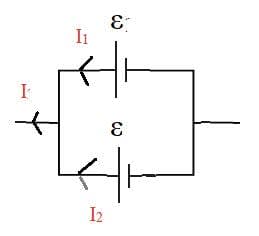
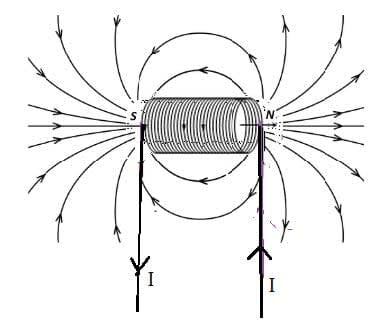
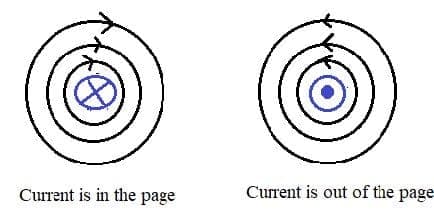
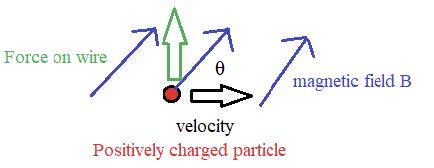
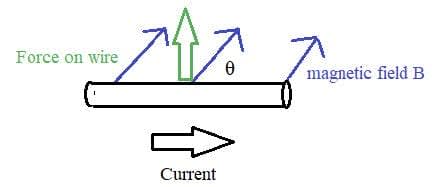
Electric cells allow us to store energy in a chemical form.
- In a battery chemical energy is converted into electrical energy. The emf is the joules of electrical energy given to each unit of charge.
- So far we have assumed that the internal resistance of the battery or power supply is negligible. We can now consider cells with an internal resistance $r$. This is due to the resistance of the components inside a battery.
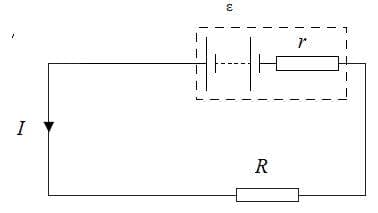
The potential difference across the battery; the terminal potential difference:
$\rm V = \varepsilon – Ir$ - The potential difference across the battery is equal to the emf when the current in the circuit is zero.
- The total electrical power by the battery $\rm = \varepsilon I$
- Electrical power dissipated in a resistor $\rm = VI = I^2R = V^2/R$
- When current is drawn from a cell, the terminal potential difference varies with time.
The terminal potential difference of a typical cell:- Falls quickly from its initial value and then stays stable for most of its lifetime.
- It then decreases rapidly to zero as the cell discharges.
- The emf and internal resistance of a cell can be determined from the following circuit:
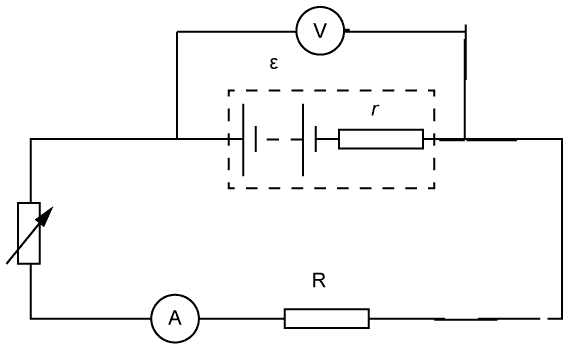
The current is varied using the variable resistor and the terminal potential difference is measured.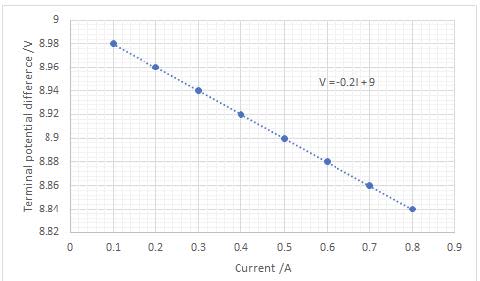
- The emf is given by the vertical intercept: in this case $\rm \varepsilon = 9.0~ V$
- The internal resistance is equal to the gradient; in this case $\rm r = 0.2~\Omega$.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA