Lewis (electron dot) structures show all the valence electrons of the atoms in the molecule or polyatomic ion. They can be used to predict molecular shape.
Shapes of Molecules
- Most atoms form a stable arrangement with eight electrons in their outer shell: the octet rule.
- There are exceptions to the octet rule:
Examples
$\rm BeCl_2, BF_3$: Be and $\rm B$ have small atomic radii and and so have less than an octet.
$\rm PCl_5, SF_6$: $\rm P$ and S are both in third period and have $\rm 3d$ orbitals in the valence energy level are available for bonding and so can have more than a stable octet. - $\bf VSEPR$ theory: the total number of electron domains determines their geometrical arrangement as they mutually repel; the shape of the molecule depends on the number of bonding pairs within this arrangement.
- The number of electron domains determines their arrangement and number of bonding pairs determine the shape.
- The angle is smaller than the symmetrical value due to the increased repulsion from a non-bong pair of electrons.
- The presence of non-boning pairs $\rm (NBP)$ or multiple bonds $\rm (MB)$ effect the bond angle as lone pairs of electrons occupy more space than bonding pairs,
- The order of repulsion between the electron pairs is:
$\rm NBP(MB) | NBP(MB)$ $>$ $\rm NBP(MB) | BP > BP | BP$
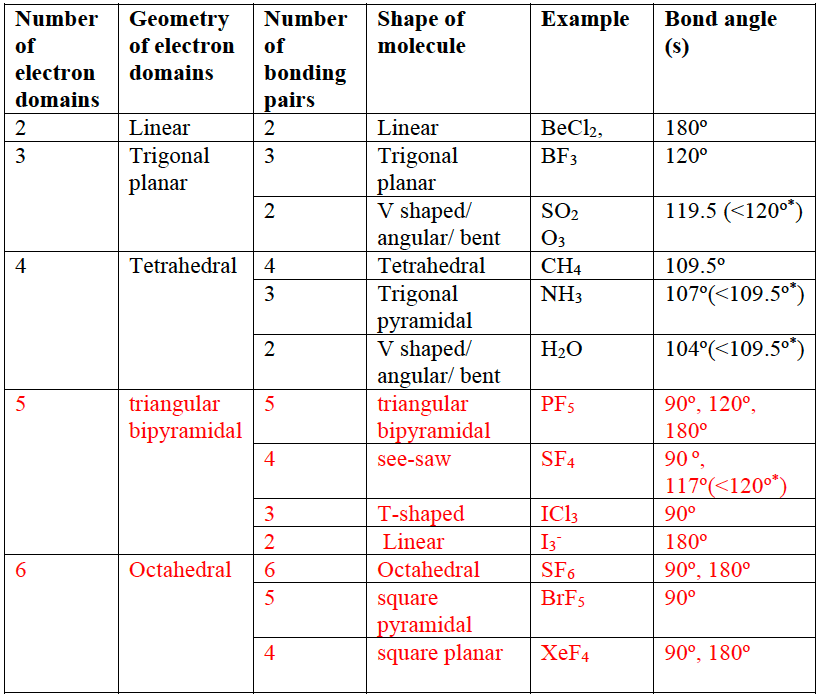
Methane, ammonia and water have four electron domains in a tetrahedral arrangement around the central atom.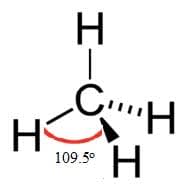 Methane
Methane
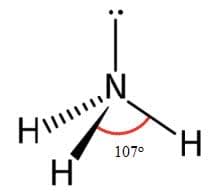 Ammonia
Ammonia
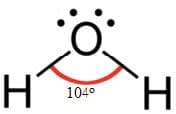
Water
- The bond angle is determined by the number of electron domains but is reduced if one of these is non-bonding pair as they have greater repulsion than a bonding pair.
Resonance Structures
- Resonance structures occur when there is more than one possible position for a double bond. This result in bond with non-integer bond orders. Ozone for example has a symmetrical structure and the bond order is $1.5$, which is intermediate between a single and double bond.
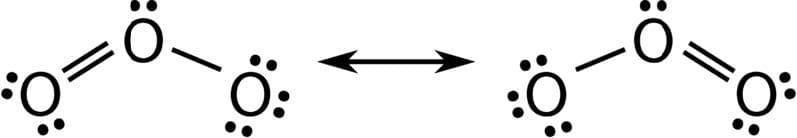 Other examples include benzene, $\rm C_6H_6$ and the ethanoate $\rm CH_3CO_2^-$ carbonate $\rm CO_3^{2-}$ and nitrate $\rm NO_3^-$ ions.
Other examples include benzene, $\rm C_6H_6$ and the ethanoate $\rm CH_3CO_2^-$ carbonate $\rm CO_3^{2-}$ and nitrate $\rm NO_3^-$ ions.
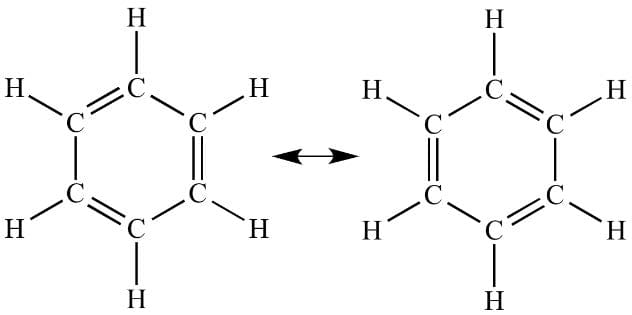 Benzene; the carbon -carbon order is $1.5$.
Benzene; the carbon -carbon order is $1.5$.
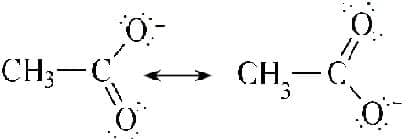 Ethanoate; the carbon-oxygen order is $1.5$.
Ethanoate; the carbon-oxygen order is $1.5$.
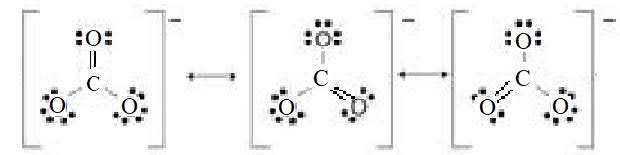 The carbonate ion. The bond order of the carbon-oxygen bond is $4/3$ and the charge on each $\rm O$ atom is $-2/3$.
The carbonate ion. The bond order of the carbon-oxygen bond is $4/3$ and the charge on each $\rm O$ atom is $-2/3$.
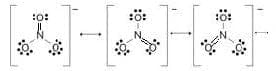 The nitrate ion. The bond order of the nitrogen-oxyegn bond is $4/3$ and the charge on each $\rm O$ atom is $-1/3$.
The nitrate ion. The bond order of the nitrogen-oxyegn bond is $4/3$ and the charge on each $\rm O$ atom is $-1/3$.
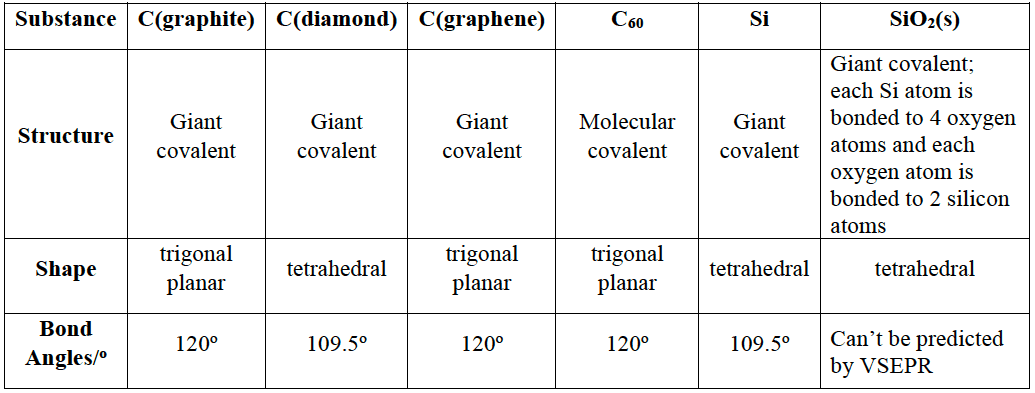
Giant Molecular Strcutures
- Carbon, silicon, and silicon dioxide form giant covalent molecules.
- Carbon occurs as allotropes with different bonding within giant molecules – diamond, graphite, fullerene, and graphene.
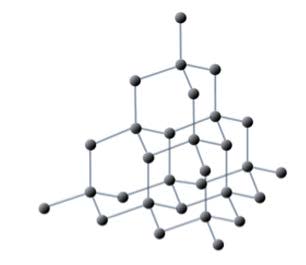 Diamond
Diamond
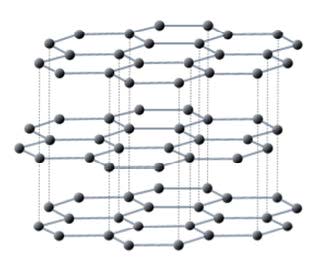
Graphite
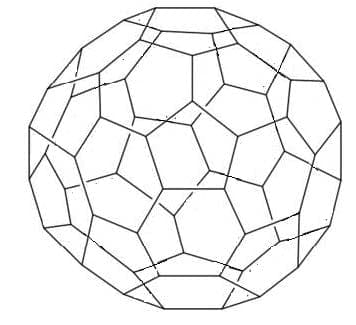
Buckminsterfullerene
which Graphene
Molecular Polarity
- The polarity of a molecule depends on:
- the polarities of its bonds which depends on the difference in electronegativities of the atoms in the bond.
- its molecular shape – symmetrical geometries can lead to cancellation between the polar bonds.

The $\rm C = O$ is polar as oxygen is more electronegative than carbon. / The $\rm Be – Cl$ is polar as chlorine is more electronegative than beryllium.
The molecules are however non-polar as the bond polarities act in opposite directions and cancel.
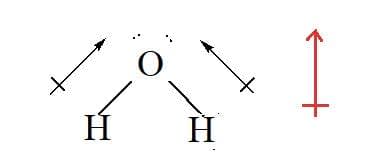
Water has polar $\rm O - H$ bonds and is a polar molecule.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
