$d$ orbitals have the same energy in an isolated atom but split into two sub-levels in a complex ion. The electric field of ligands cause the $d$ orbitals in complex ions to split so that the energy of an electron transition between them corresponds to a photon of visible light
Colour and $\bf d$ orbitals.
- $\rm d$ orbitals have the same energy in an isolated atom but split into two sub-levels in a complex ion. In an octahedral complex, for example, electrons in three of the $\rm d$ orbitals which are orientated between the axis will experience less repulsion from the lone pairs of electrons of the ligands than the orbitals which are orientated along the axis. The three orbitals which experience less repulsion are of lower energy.
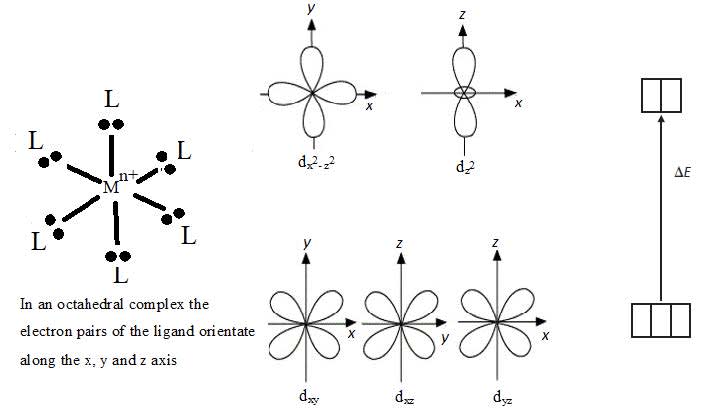
- The electric field of ligands cause the $\rm d$ orbitals in complex ions to split so that the energy of an electron transition between them corresponds to a photon of visible light.
- Transition metal ions are coloured due to $\bf d–d$ electron transitions between d orbitals which are split in the electric field due to the presence of the lone pairs of the ligands.
- The colour observed is complementary to the colour absorbed and can be deduced from the colour wheel.
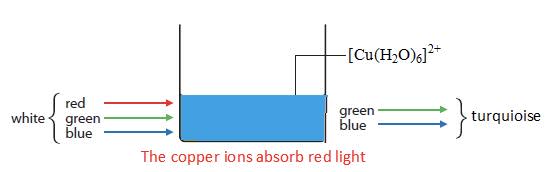
A simplified colour wheel - if red light is absorbed the complementary colour, a mix of blue and green, turquoise is transmitted.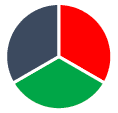
- The colour of a complex depends on the identity of the metal ion, the oxidation state of the metal, and the identity of the ligand.
- Ions with higher charge and ligands with greater charge density produce a greater split in the $\rm d$ orbitals.
- The spectrochemical series arranges the ligands according to the energy separation between the two sets of $\rm d$ orbitals.
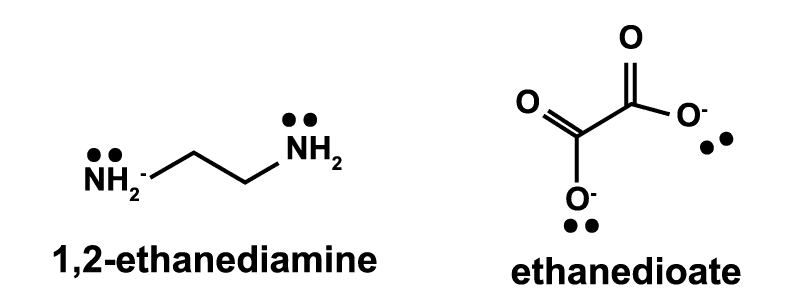
$\rm I^- < Br^- < S^{2-} < Cl^- < F^- < OH^- <$ $\rm H_2O < SCN^- < NH_3 < CN^- \approx CO$ - Polydentate ligands form more than one coordinate bond with the metal ion. For example, $1,2$-ethanediamine and ethanediooate are both bidentate ligands as they both form two coordinate bonds with a metal ion.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
