Energy is absorbed when bonds are broken and is released when bonds are formed.
- Average bond energy is the energy required to break one mole of the same type of bonds in the gaseous state averaged over a variety of similar compounds.
- Bond breaking absorbs energy and is endothermic. Bond making releases energy and is exothermic.
$\rm \Delta H_{reaction} = \sum E_{bonds~broken}$ $-$ $\rm\sum E_{bonds~formed}$
When $\rm \sum E_{bonds~broken} > \sum E_{bonds~formed}$ : the reaction is endothermic.
When $\rm\sum E_{bonds~formed} > \sum E_{bonds~broken}$ : the reaction is exothermic.
This calculations does not agree precisely with experimental values as bond energies are average values.
- Enthalpy of reactions calculated from bond enthalpies do not include intermolecular interaction and so are only accurate for reactions in the gaseous state.
- The bond order in ozone is $1.5$ The bond order in oxygen is $2$. The bonds in ozone $\rm O_3$ are weaker than those in oxygen $\rm O_2$ and are broken by $\rm UV$ light of longer wavelengths.
- The ozone cycle describes how ozone is both formed and depleted by natural processes in the atmosphere.
Worked Example
(a) Determine the standard enthalpy change for the combustion of propane using the given bond enthalpy.
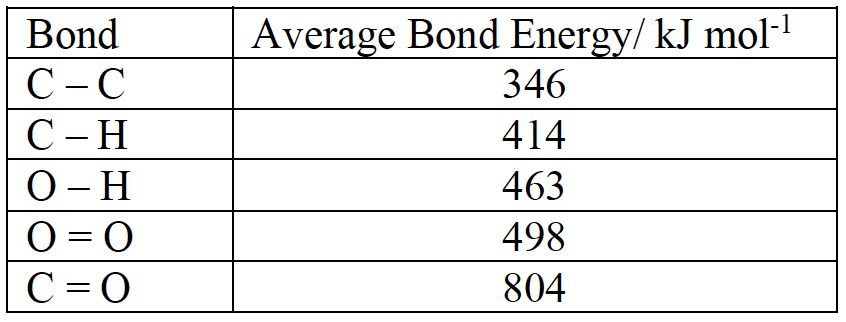
Solution
Start with a balanced chemical equation showing all the bonds:
$\begin{array}{ccc}& & \rm H && \rm H && \rm H\\
&& | && | && | \\
\rm H & — & \rm C & — & \rm C & — & \rm C & — & \rm H(g)\\
&& | && | && |\\
&&\rm H && \rm H && \rm H\\
\end{array}$ $\begin{array}{cc}+ & \rm 5 O & = & \rm O(g)\end{array}$ $\rightarrow$ $\begin{array}{cc} \rm 3 O & = & \rm C & = & \rm O(g) & + & \rm 4O & — & \rm H (l)\\
&&&&&|\\
&&&&&\rm H\end{array}$
Identify the bond broken formed and broken and the corresponding enthalpy changes:
![]()
![]()
Energy required for bond breaking:
$\rm \Delta H^o_c = \sum(BE_{ bonds~broken})$ $-$ $\rm \sum(BE_{bonds~formed})$
$\rm \Delta H^o_c = 6~494 - 8~528$
$\rm Enthalpy~change = -2~034~kJ~mol^{-1}$
(b) The experimental value for the standard enthalpy change for the complete combustion of propane is $\rm −2~219~kJ~mol^{-1}$. Discuss the difference between the two values.
The difference can be explained by two factors:
- bond enthalpy calculations do not include intermolecular interactions. Water is in the liquid state.
- bond enthalpy values are average values and may be different from the bond energies in the specific substances.
 Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
Nouveau ! Découvrez Nomad'IA : le savoir de nos 400 profs + la magie de l'IA
